Endo-lysosomal Aβ concentration and pH trigger formation of Aβ oligomers that potently induce Tau missorting
- PMID: 34330900
- PMCID: PMC8324842
- DOI: 10.1038/s41467-021-24900-4
Endo-lysosomal Aβ concentration and pH trigger formation of Aβ oligomers that potently induce Tau missorting
Abstract
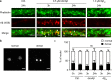
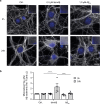
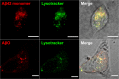
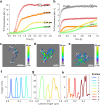
Amyloid-β peptide (Aβ) forms metastable oligomers >50 kDa, termed AβOs, that are more effective than Aβ amyloid fibrils at triggering Alzheimer's disease-related processes such as synaptic dysfunction and Tau pathology, including Tau mislocalization. In neurons, Aβ accumulates in endo-lysosomal vesicles at low pH. Here, we show that the rate of AβO assembly is accelerated 8,000-fold upon pH reduction from extracellular to endo-lysosomal pH, at the expense of amyloid fibril formation. The pH-induced promotion of AβO formation and the high endo-lysosomal Aβ concentration together enable extensive AβO formation of Aβ42 under physiological conditions. Exploiting the enhanced AβO formation of the dimeric Aβ variant dimAβ we furthermore demonstrate targeting of AβOs to dendritic spines, potent induction of Tau missorting, a key factor in tauopathies, and impaired neuronal activity. The results suggest that the endosomal/lysosomal system is a major site for the assembly of pathomechanistically relevant AβOs.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
The Co-oligomers of Aβ42 and Human Islet Amyloid Polypeptide Exacerbate Neurotoxicity and Alzheimer-like Pathology at Cellular Level.Neuroscience. 2024 May 24;547:37-55. doi: 10.1016/j.neuroscience.2024.04.002. Epub 2024 Apr 9. Neuroscience. 2024. PMID: 38604526
-
Amyloid β oligomers in Alzheimer's disease pathogenesis, treatment, and diagnosis.Acta Neuropathol. 2015 Feb;129(2):183-206. doi: 10.1007/s00401-015-1386-3. Epub 2015 Jan 22. Acta Neuropathol. 2015. PMID: 25604547 Free PMC article. Review.
-
Linking amyloid-β and tau: amyloid-β induced synaptic dysfunction via local wreckage of the neuronal cytoskeleton.Neurodegener Dis. 2012;10(1-4):64-72. doi: 10.1159/000332816. Epub 2011 Dec 7. Neurodegener Dis. 2012. PMID: 22156588
-
Intracellular amyloid β oligomers impair organelle transport and induce dendritic spine loss in primary neurons.Acta Neuropathol Commun. 2015 Aug 21;3:51. doi: 10.1186/s40478-015-0230-2. Acta Neuropathol Commun. 2015. PMID: 26293809 Free PMC article.
-
Oligomerization and Conformational Change Turn Monomeric β-Amyloid and Tau Proteins Toxic: Their Role in Alzheimer's Pathogenesis.Molecules. 2020 Apr 3;25(7):1659. doi: 10.3390/molecules25071659. Molecules. 2020. PMID: 32260279 Free PMC article. Review.
Cited by
-
Isoform- and cell-state-specific APOE homeostasis and function.Neural Regen Res. 2024 Nov 1;19(11):2456-2466. doi: 10.4103/NRR.NRR-D-23-01470. Epub 2024 Jan 31. Neural Regen Res. 2024. PMID: 38526282 Free PMC article.
-
Negative Regulation of Cathepsins by β-Amyloid.eNeuro. 2024 Jan 10;11(1):ENEURO.0258-23.2023. doi: 10.1523/ENEURO.0258-23.2023. Print 2024 Jan. eNeuro. 2024. PMID: 38199815 Free PMC article.
-
Yin-Yang: two sides of extracellular vesicles in inflammatory diseases.J Nanobiotechnology. 2024 Aug 27;22(1):514. doi: 10.1186/s12951-024-02779-9. J Nanobiotechnology. 2024. PMID: 39192300 Free PMC article. Review.
-
MAD-microbial (origin of) Alzheimer's disease hypothesis: from infection and the antimicrobial response to disruption of key copper-based systems.Front Neurosci. 2024 Oct 2;18:1467333. doi: 10.3389/fnins.2024.1467333. eCollection 2024. Front Neurosci. 2024. PMID: 39416952 Free PMC article. Review.
-
A Disulfide-Stabilized Aβ that Forms Dimers but Does Not Form Fibrils.Biochemistry. 2022 Feb 15;61(4):252-264. doi: 10.1021/acs.biochem.1c00739. Epub 2022 Jan 26. Biochemistry. 2022. PMID: 35080857 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

