Toxin import through the antibiotic efflux channel TolC
- PMID: 34330923
- PMCID: PMC8324772
- DOI: 10.1038/s41467-021-24930-y
Toxin import through the antibiotic efflux channel TolC
Abstract
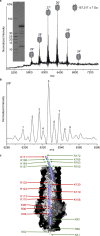
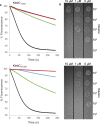
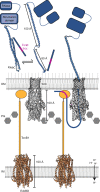
Bacteria often secrete diffusible protein toxins (bacteriocins) to kill bystander cells during interbacterial competition. Here, we use biochemical, biophysical and structural analyses to show how a bacteriocin exploits TolC, a major outer-membrane antibiotic efflux channel in Gram-negative bacteria, to transport itself across the outer membrane of target cells. Klebicin C (KlebC), a rRNase toxin produced by Klebsiella pneumoniae, binds TolC of a related species (K. quasipneumoniae) with high affinity through an N-terminal, elongated helical hairpin domain common amongst bacteriocins. The KlebC helical hairpin opens like a switchblade to bind TolC. A cryo-EM structure of this partially translocated state, at 3.1 Å resolution, reveals that KlebC associates along the length of the TolC channel. Thereafter, the unstructured N-terminus of KlebC protrudes beyond the TolC iris, presenting a TonB-box sequence to the periplasm. Association with proton-motive force-linked TonB in the inner membrane drives toxin import through the channel. Finally, we demonstrate that KlebC binding to TolC blocks drug efflux from bacteria. Our results indicate that TolC, in addition to its known role in antibiotic export, can function as a protein import channel for bacteriocins.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

