Autoantibodies against NMDA receptor 1 modify rather than cause encephalitis
- PMID: 34331009
- PMCID: PMC8872987
- DOI: 10.1038/s41380-021-01238-3
Autoantibodies against NMDA receptor 1 modify rather than cause encephalitis
Abstract
The etiology and pathogenesis of "anti-N-methyl-D-aspartate-receptor (NMDAR) encephalitis" and the role of autoantibodies (AB) in this condition are still obscure. While NMDAR1-AB exert NMDAR-antagonistic properties by receptor internalization, no firm evidence exists to date that NMDAR1-AB by themselves induce brain inflammation/encephalitis. NMDAR1-AB of all immunoglobulin classes are highly frequent across mammals with multiple possible inducers and boosters. We hypothesized that "NMDAR encephalitis" results from any primary brain inflammation coinciding with the presence of NMDAR1-AB, which may shape the encephalitis phenotype. Thus, we tested whether following immunization with a "cocktail" of 4 NMDAR1 peptides, induction of a spatially and temporally defined sterile encephalitis by diphtheria toxin-mediated ablation of pyramidal neurons ("DTA" mice) would modify/aggravate the ensuing phenotype. In addition, we tried to replicate a recent report claiming that immunizing just against the NMDAR1-N368/G369 region induced brain inflammation. Mice after DTA induction revealed a syndrome comprising hyperactivity, hippocampal learning/memory deficits, prefrontal cortical network dysfunction, lasting blood brain-barrier impairment, brain inflammation, mainly in hippocampal and cortical regions with pyramidal neuronal death, microgliosis, astrogliosis, modest immune cell infiltration, regional atrophy, and relative increases in parvalbumin-positive interneurons. The presence of NMDAR1-AB enhanced the hyperactivity (psychosis-like) phenotype, whereas all other readouts were identical to control-immunized DTA mice. Non-DTA mice with or without NMDAR1-AB were free of any encephalitic signs. Replication of the reported NMDAR1-N368/G369-immunizing protocol in two large independent cohorts of wild-type mice completely failed. To conclude, while NMDAR1-AB can contribute to the behavioral phenotype of an underlying encephalitis, induction of an encephalitis by NMDAR1-AB themselves remains to be proven.
© 2021. The Author(s).
Conflict of interest statement
WS is a member of the board and holds stocks in Euroimmun AG. All other authors declare no competing financial or other interests.
Figures
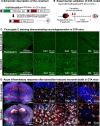
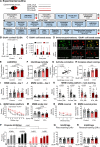
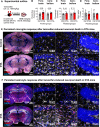
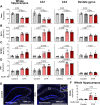
References
-
- Mikasova L, De Rossi P, Bouchet D, Georges F, Rogemond V, Didelot A, et al. Disrupted surface cross-talk between NMDA and Ephrin-B2 receptors in anti-NMDA encephalitis. Brain. 2012;135:1606–21. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

