Spared Nerve Injury Causes Sexually Dimorphic Mechanical Allodynia and Differential Gene Expression in Spinal Cords and Dorsal Root Ganglia in Rats
- PMID: 34331199
- PMCID: PMC8497331
- DOI: 10.1007/s12035-021-02447-1
Spared Nerve Injury Causes Sexually Dimorphic Mechanical Allodynia and Differential Gene Expression in Spinal Cords and Dorsal Root Ganglia in Rats
Abstract
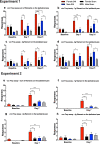
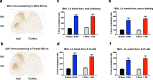
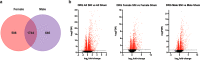
Neuropathic pain is more prevalent in women. However, females are under-represented in animal experiments, and the mechanisms of sex differences remain inadequately understood. We used the spared nerve injury (SNI) model in rats to characterize sex differences in pain behaviour, unbiased RNA-Seq and proteomics to study the mechanisms. Male and female rats were subjected to SNI- and sham-surgery. Mechanical and cold allodynia were assessed. Ipsilateral lumbar dorsal root ganglia (DRG) and spinal cord (SC) segments were collected for RNA-seq analysis with DESeq2 on Day 7. Cerebrospinal fluid (CSF) samples for proteomic analysis and DRGs and SCs for analysis of IB-4 and CGRP, and IBA1 and GFAP, respectively, were collected on Day 21. Females developed stronger mechanical allodynia. There were no differences between the sexes in CGRP and IB-4 in the DRG or glial cell markers in the SC. No CSF protein showed change following SNI. DRG and SC showed abundant changes in gene expression. Sexually dimorphic responses were found in genes related to T-cells (cd28, ctla4, cd274, cd4, prf1), other immunological responses (dpp4, c5a, cxcr2 and il1b), neuronal transmission (hrh3, thbs4, chrna4 and pdyn), plasticity (atf3, c1qc and reg3b), and others (bhlhe22, mcpt1l, trpv6). We observed significantly stronger mechanical allodynia in females and numerous sexually dimorphic changes in gene expression following SNI in rats. Several genes have previously been linked to NP, while some are novel. Our results suggest gene targets for further studies in the development of new, possibly sex-specific, therapies for NP.
Keywords: Behaviour; Neuropathic pain; Proteomics; Rat; Sex-differences; Transcriptomics.
© 2021. The Author(s).
Conflict of interest statement
Eija Kalso has received consultant honoraria from Orion Pharma and Pfizer. The consultancy is indirectly related to the submitted work. The authors have no other conflicts of interest to declare that are relevant to the content of this article.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

