Brain Temperature Influences Intracranial Pressure and Cerebral Perfusion Pressure After Traumatic Brain Injury: A CENTER-TBI Study
- PMID: 34331210
- PMCID: PMC8692292
- DOI: 10.1007/s12028-021-01294-1
Brain Temperature Influences Intracranial Pressure and Cerebral Perfusion Pressure After Traumatic Brain Injury: A CENTER-TBI Study
Abstract
Background: After traumatic brain injury (TBI), fever is frequent. Brain temperature (BT), which is directly linked to body temperature, may influence brain physiology. Increased body and/or BT may cause secondary brain damage, with deleterious effects on intracranial pressure (ICP), cerebral perfusion pressure (CPP), and outcome.
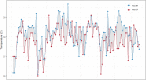
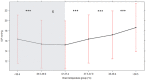
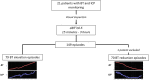
Methods: Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI), a prospective multicenter longitudinal study on TBI in Europe and Israel, includes a high resolution cohort of patients with data sampled at a high frequency (from 100 to 500 Hz). In this study, simultaneous BT, ICP, and CPP recordings were investigated. A mixed-effects linear model was used to examine the association between different BT levels and ICP. We additionally focused on changes in ICP and CPP during the episodes of BT changes (Δ BT ≥ 0.5 °C lasting from 15 min to 3 h) up or downward. The significance of ICP and CPP variations was estimated with the paired samples Wilcoxon test (also known as Wilcoxon signed-rank test).
Results: Twenty-one patients with 2,435 h of simultaneous BT and ICP monitoring were studied. All patients reached a BT of 38 °C and experienced at least one episode of ICP above 20 mm Hg. The linear mixed-effects model revealed an association between BT above 37.5 °C and higher ICP levels that was not confirmed for lower BT. We identified 149 episodes of BT changes. During BT elevations (n = 79) ICP increased, whereas CPP was reduced; opposite ICP and CPP variations occurred during episodes of BT reduction (n = 70). All these changes were of moderate clinical relevance (increase of ICP of 4.5 and CPP decrease of 7.5 mm Hg for BT rise, and ICP reduction of 1.7 and CPP elevation of 3.7 mm Hg during BT defervescence), even if statistically significant (p < 0.0001). It has to be noted, however, that a number of therapeutic interventions against intracranial hypertension was documented during those episodes.
Conclusions: Patients after TBI usually develop BT > 38 °C soon after the injury. BT may influence brain physiology, as reflected by ICP and CPP. An association between BT exceeding 37.5 °C and a higher ICP was identified but not confirmed for lower BT ranges. The relationship between BT, ICP, and CPP become clearer during rapid temperature changes. During episodes of temperature elevation, BT seems to have a significant impact on ICP and CPP.
Keywords: Brain temperature; Cerebral perfusion pressure; Fever; Hyperthermia; Intracranial pressure; Neuromonitoring; Traumatic brain injury.
© 2021. The Author(s).
Conflict of interest statement
Dr. Birg reports grants from the European Commission 7th Framework program (602150), grants from the Hannelore Kohl Stiftung (Germany), grants from OneMind (USA), grants from Integra LifeSciences Corporation (USA), and grants from Neurotrauma Sciences (USA) during the conduct of the study. Dr. Ortolano reports grants from the European Commission 7th Framework program (602150), grants from the Hannelore Kohl Stiftung (Germany), grants from OneMind (USA), grants from Integra LifeSciences Corporation (USA), and grants from Neurotrauma Sciences (USA) during the conduct of the study. Dr. Wiegers reports grants from the European Commission 7th Framework program (602150), grants from the Hannelore Kohl Stiftung (Germany), grants from OneMind (USA), grants from Integra LifeSciences Corporation (USA), and grants from Neurotrauma Sciences (USA) during the conduct of the study. Dr. Smielewski reports grants from the European Commission 7th Framework program (602150) during the conduct of the study; personal fees from Cambridge Enterprise Ltd, Cambridge, UK, outside the submitted work. Dr. Savchenko reports grants from the European Commission 7th Framework program (602150), grants from the Hannelore Kohl Stiftung (Germany), grants from OneMind (USA), grants from Integra LifeSciences Corporation (USA), and grants from Neurotrauma Sciences (USA) during the conduct of the study. Dr. Ianosi reports grants from the European Commission 7th Framework program (602150), grants from the Hannelore Kohl Stiftung (Germany), grants from OneMind (USA), grants from Integra LifeSciences Corporation (USA), grants from Neurotrauma Sciences (USA), during the conduct of the study. Dr. Helbok reports grants from the European Commission 7th Framework program (602150), grants from the Hannelore Kohl Stiftung (Germany), grants from OneMind (USA), grants from Integra LifeSciences Corporation (USA), grants from Neurotrauma Sciences (USA), during the conduct of the study. Dr. Rossi reports grants from the European Commission 7th Framework program (602150), grants from the Hannelore Kohl Stiftung (Germany), grants from OneMind (USA), grants from Integra LifeSciences Corporation (USA), grants from Neurotrauma Sciences (USA), during the conduct of the study. Dr. Carbonara reports grants from the European Commission 7th Framework program (602150), grants from the Hannelore Kohl Stiftung (Germany), grants from OneMind (USA), grants from Integra LifeSciences Corporation (USA), grants from Neurotrauma Sciences (USA), during the conduct of the study. Dr. Zoerle reports grants from the European Commission 7th Framework program (602150), grants from the Hannelore Kohl Stiftung (Germany), grants from OneMind (USA), grants from Integra LifeSciences Corporation (USA), grants from Neurotrauma Sciences (USA), during the conduct of the study. Dr. Stochetti reports grants from the European Commission 7th Framework program (602150), grants from the Hannelore Kohl Stiftung (Germany), grants from OneMind (USA), grants from Integra LifeSciences Corporation (USA), grants from Neurotrauma Sciences (USA), during the conduct of the study.
Figures


Comment in
-
Commentary on "Brain Temperature Influences Intracranial Pressure and Cerebral Perfusion Pressure After Traumatic Brain Injury".Neurocrit Care. 2021 Dec;35(3):615-616. doi: 10.1007/s12028-021-01295-0. Epub 2021 Jul 30. Neurocrit Care. 2021. PMID: 34331209 Free PMC article. No abstract available.
References
-
- Dietrich W, Alonso O, Halley M, et al. Delayed posttraumatic brain hyperthermia worsens outcome after fluid percussion brain injury: a light and electron microscopic study in rats. Neurosurgery. 1996;38(3):533–541. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

