Multiplex Imaging of Rho GTPase Activities in Living Cells
- PMID: 34331278
- PMCID: PMC8367095
- DOI: 10.1007/978-1-0716-1593-5_4
Multiplex Imaging of Rho GTPase Activities in Living Cells
Abstract
Förster resonance energy transfer (FRET) biosensors are popular and useful for directly observing cellular signaling pathways in living cells. Until recently, multiplex imaging of genetically encoded FRET biosensors to simultaneously monitor several protein activities in one cell was limited due to a lack of spectrally compatible FRET pair of fluorescent proteins. With the recent development of miRFP series of near-infrared (NIR) fluorescent proteins, we are now able to extend the spectrum of FRET biosensors beyond blue-green-yellow into NIR. These new NIR FRET biosensors enable direct multiplex imaging together with commonly used cyan-yellow FRET biosensors. We describe herein a method to produce cell lines harboring two compatible FRET biosensors. We will then discuss how to directly multiplex-image these FRET biosensors in living cells. The approaches described herein are generally applicable to any combinations of genetically encoded, ratiometric FRET biosensors utilizing the cyan-yellow and NIR fluorescence.
Keywords: FRET biosensor; Multiplex imaging; Near-infrared fluorescent protein; Rho GTPases.
© 2021. Springer Science+Business Media, LLC, part of Springer Nature.
Figures


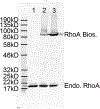


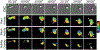
Similar articles
-
Unravelling molecular dynamics in living cells: Fluorescent protein biosensors for cell biology.J Microsc. 2025 May;298(2):123-184. doi: 10.1111/jmi.13270. Epub 2024 Feb 15. J Microsc. 2025. PMID: 38357769 Review.
-
Characterization of Genetically Encoded FRET Biosensors for Rho-Family GTPases.Methods Mol Biol. 2018;1821:87-106. doi: 10.1007/978-1-4939-8612-5_7. Methods Mol Biol. 2018. PMID: 30062407 Free PMC article.
-
Direct multiplex imaging and optogenetics of Rho GTPases enabled by near-infrared FRET.Nat Chem Biol. 2018 Jun;14(6):591-600. doi: 10.1038/s41589-018-0044-1. Epub 2018 Apr 23. Nat Chem Biol. 2018. PMID: 29686359 Free PMC article.
-
Booster, a Red-Shifted Genetically Encoded Förster Resonance Energy Transfer (FRET) Biosensor Compatible with Cyan Fluorescent Protein/Yellow Fluorescent Protein-Based FRET Biosensors and Blue Light-Responsive Optogenetic Tools.ACS Sens. 2020 Mar 27;5(3):719-730. doi: 10.1021/acssensors.9b01941. Epub 2020 Feb 26. ACS Sens. 2020. PMID: 32101394
-
A practical method for monitoring FRET-based biosensors in living animals using two-photon microscopy.Am J Physiol Cell Physiol. 2015 Dec 1;309(11):C724-35. doi: 10.1152/ajpcell.00182.2015. Epub 2015 Sep 2. Am J Physiol Cell Physiol. 2015. PMID: 26333599 Free PMC article. Review.
Cited by
-
Seeing Neurodegeneration in a New Light Using Genetically Encoded Fluorescent Biosensors and iPSCs.Int J Mol Sci. 2023 Jan 16;24(2):1766. doi: 10.3390/ijms24021766. Int J Mol Sci. 2023. PMID: 36675282 Free PMC article. Review.
-
ANKS1B encoded AIDA-1 regulates social behaviors by controlling oligodendrocyte function.Nat Commun. 2023 Dec 21;14(1):8499. doi: 10.1038/s41467-023-43438-1. Nat Commun. 2023. PMID: 38129387 Free PMC article.
-
Unravelling molecular dynamics in living cells: Fluorescent protein biosensors for cell biology.J Microsc. 2025 May;298(2):123-184. doi: 10.1111/jmi.13270. Epub 2024 Feb 15. J Microsc. 2025. PMID: 38357769 Review.
-
Fidgetin-like 2 depletion enhances cell migration by regulating GEF-H1, RhoA, and FAK.Biophys J. 2023 Sep 19;122(18):3600-3610. doi: 10.1016/j.bpj.2022.12.018. Epub 2022 Dec 15. Biophys J. 2023. PMID: 36523161 Free PMC article.
-
Comparison of fluorescence lifetime and multispectral imaging for quantitative multiplexing in biological tissue.Biomed Opt Express. 2022 Jun 9;13(7):3854-3868. doi: 10.1364/BOE.459935. eCollection 2022 Jul 1. Biomed Opt Express. 2022. PMID: 35991924 Free PMC article.
References
-
- Pertz O, Hodgson L, Klemke RL, Hahn KM (2006) Spatiotemporal dynamics of RhoA activity in migrating cells. Nature 440 (7087):1069–1072 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

