Know your enemy or find your friend?-Induction of IgA at mucosal surfaces
- PMID: 34331314
- PMCID: PMC7612940
- DOI: 10.1111/imr.13014
Know your enemy or find your friend?-Induction of IgA at mucosal surfaces
Abstract
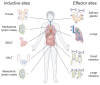
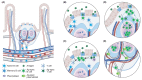
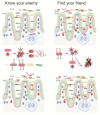
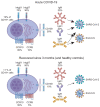
Most antibodies produced in the body are of the IgA class. The dominant cell population producing them are plasma cells within the lamina propria of the gastrointestinal tract, but many IgA-producing cells are also found in the airways, within mammary tissues, the urogenital tract and inside the bone marrow. Most IgA antibodies are transported into the lumen by epithelial cells as part of the mucosal secretions, but they are also present in serum and other body fluids. A large part of the commensal microbiota in the gut is covered with IgA antibodies, and it has been demonstrated that this plays a role in maintaining a healthy balance between the host and the bacteria. However, IgA antibodies also play important roles in neutralizing pathogens in the gastrointestinal tract and the upper airways. The distinction between the two roles of IgA - protective and balance-maintaining - not only has implications on function but also on how the production is regulated. Here, we discuss these issues with a special focus on gut and airways.
Keywords: IgA; commensal microbiota; infection; mucosa.
© 2021 The Authors. Immunological Reviews published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors do not declare any conflicts of interest.
Figures
References
-
- Pabst R, Russell MW, Brandtzaeg P. Tissue distribution of lymphocytes and plasma cells and the role of the gut. Trends Immunol. 2008;29:206–208. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

