Synthesis and Antiprotozoal Profile of 3,4,5-Trisubstituted Isoxazoles
- PMID: 34331350
- PMCID: PMC8485799
- DOI: 10.1002/open.202100141
Synthesis and Antiprotozoal Profile of 3,4,5-Trisubstituted Isoxazoles
Abstract
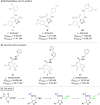
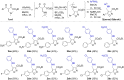
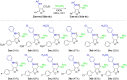
A series of 60 4-aminomethyl 5-aryl-3-substituted isoxazoles were synthesized by an efficient method and evaluated in vitro against Leishmania amazonensis and Trypanosoma cruzi, protozoa that cause the neglected tropical diseases leishmaniasis and Chagas disease, respectively. Thirteen compounds exhibited a selective index greater than 10. The series of 3-N-acylhydrazone isoxazole derivatives bearing the bithiophene core exhibited the best antiparasitic effects.
Keywords: N-acylhydrazone derivatives; antileishmanial drugs; antiprotozoal agents; cyclocondensation reactions; isoxazoles.
© 2021 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Design and synthesis of a new series of 3,5-disubstituted isoxazoles active against Trypanosoma cruzi and Leishmania amazonensis.Eur J Med Chem. 2017 Mar 10;128:25-35. doi: 10.1016/j.ejmech.2017.01.029. Epub 2017 Jan 23. Eur J Med Chem. 2017. PMID: 28152426
-
Antiparasitic activities of novel pyrimidine N-acylhydrazone hybrids.Drug Dev Res. 2021 Apr;82(2):230-240. doi: 10.1002/ddr.21745. Epub 2020 Sep 30. Drug Dev Res. 2021. PMID: 32996619
-
Exploring microalgal and cyanobacterial metabolites with antiprotozoal activity against Leishmania and Trypanosoma parasites.Acta Trop. 2024 Mar;251:107116. doi: 10.1016/j.actatropica.2023.107116. Epub 2023 Dec 28. Acta Trop. 2024. PMID: 38159713 Review.
-
In vitro evaluation of arylsubstituted imidazoles derivatives as antiprotozoal agents and docking studies on sterol 14α-demethylase (CYP51) from Trypanosoma cruzi, Leishmania infantum, and Trypanosoma brucei.Parasitol Res. 2019 May;118(5):1533-1548. doi: 10.1007/s00436-019-06206-z. Epub 2019 Mar 23. Parasitol Res. 2019. PMID: 30903349
-
Leishmanicidal and Trypanocidal Activity of Metal Complexes with 1,2,4-Triazolo[1,5-a]pyrimidines: Insights on their Therapeutic Potential against Leishmaniasis and Chagas Disease.Curr Med Chem. 2017;24(25):2796-2806. doi: 10.2174/0929867324666170516122024. Curr Med Chem. 2017. PMID: 28521698 Review.
Cited by
-
Hydroxyalkyne-Bithiophene Derivatives: Synthesis and Antileishmanial Activity.Chem Biol Drug Des. 2025 Aug;106(2):e70167. doi: 10.1111/cbdd.70167. Chem Biol Drug Des. 2025. PMID: 40847477 Free PMC article.
References
-
- Santos S. S., de Araújo R. V., Giarolla J., El Seoud O., Ferreira E. I., Int. J. Antimicrob. Agents 2020, 55, 105906. - PubMed
-
- Corpas-López V., Tabraue-Chávez M., Sixto-López Y., Panadero-Fajardo S., Alves De Lima Franco F., Domínguez-Seglar J. F., Morillas-Márquez F., Franco-Montalbán F., Díaz-Gavilán M., Correa-Basurto J., López-Viota J., López-Viota M., Pérez Del Palacio J., De La Cruz M., De Pedro N., Martín-Sánchez J., Gómez-Vidal J. A., J. Med. Chem. 2020, 63, 5734–5751. - PubMed
-
- None
-
- WHO, World Health Organization fact sheet on Leishmaniasis, https://www.who.int/en/news-room/fact-sheets/detail/leishmaniasis, 2 March 2020 (accessed on 5 April 2021);
-
- WHO, World Health Organization fact sheet on Chagas disease, https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(america..., 11 March 2020 (accessed on 5 April 2021).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

