Identification of the prognostic value of elevated ANGPTL4 expression in gallbladder cancer-associated fibroblasts
- PMID: 34331381
- PMCID: PMC8419759
- DOI: 10.1002/cam4.4150
Identification of the prognostic value of elevated ANGPTL4 expression in gallbladder cancer-associated fibroblasts
Abstract
Background: Cancer-associated fibroblasts (CAFs) with different gene profiles from normal fibroblasts (NFs) have been implicated in tumor progression. Angiopoietin-like protein 4 (ANGPTL4) has been shown to regulate tumor angiogenesis and metastasis, and predict poor prognosis. However, the ANGPTL4 expression in CAFs, especially in gallbladder CAFs (GCAFs) and its relationship with patient prognosis is unclear.
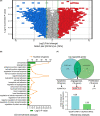
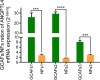
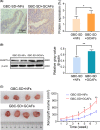
Methods: Affymetrix gene profile chip analysis in vitro was performed to detect the different gene expression profiles between GCAFs and NFs. RT-qPCR, immunohistochemistry, and western blotting were performed to investigate the different expression levels of ANGPTL4 in GCAFs/NFs in vitro and in an in vivo nude mouse model of xenograft tumors. Finally, the ANGPTL4 expression was investigated in the stroma of different lesion tissues of the human gallbladder by immunohistochemistry, especially the expression in GCAFs in vivo by co-immunofluorescence, and their prognostic significance in patients with gallbladder cancer (GBC) was assessed.
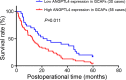
Results: ANGPTL4 was upregulated in both GCAFs in vitro and in the xenograft stroma of nude mice in vivo, and its expression was also significantly upregulated in human GBC stroma co-localized with the interstitial markers fibroblast secreted protein-1 and α-smooth muscle actin. In addition, the elevated ANGPTL4 expression in GCAFs was correlated with tumor differentiation, liver metastasis, venous invasion and Nevin staging, and GBC patients with an elevated ANGPTL4 expression in GACFs were found to have a lower survival rate.
Conclusions: Increased ANGPTL4 expression in GCAFs correlates with poor patient prognosis, which indicates a potential therapeutic target for human GBCs.
Keywords: ANGPTL4; cancer-associated fibroblast; gallbladder cancer; prognosis.
© 2021 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no conflict of interests.
Figures



Similar articles
-
Emerging roles of angiopoietin‑like 4 in human tumors (Review).Int J Oncol. 2025 Feb;66(2):9. doi: 10.3892/ijo.2024.5715. Epub 2024 Dec 20. Int J Oncol. 2025. PMID: 39704206 Free PMC article. Review.
-
Gallbladder cancer-associated fibroblasts promote vasculogenic mimicry formation and tumor growth in gallbladder cancer via upregulating the expression of NOX4, a poor prognosis factor, through IL-6-JAK-STAT3 signal pathway.J Exp Clin Cancer Res. 2020 Nov 5;39(1):234. doi: 10.1186/s13046-020-01742-4. J Exp Clin Cancer Res. 2020. PMID: 33153467 Free PMC article.
-
Upregulated NOX1 expression in gallbladder cancer‑associated fibroblasts predicts a poor prognosis.Oncol Rep. 2019 Oct;42(4):1475-1486. doi: 10.3892/or.2019.7249. Epub 2019 Jul 25. Oncol Rep. 2019. PMID: 31364740
-
Thrombospondin 4/integrin α2/HSF1 axis promotes proliferation and cancer stem-like traits of gallbladder cancer by enhancing reciprocal crosstalk between cancer-associated fibroblasts and tumor cells.J Exp Clin Cancer Res. 2021 Jan 6;40(1):14. doi: 10.1186/s13046-020-01812-7. J Exp Clin Cancer Res. 2021. PMID: 33407730 Free PMC article.
-
Potential role of ANGPTL4 in cancer progression, metastasis, and metabolism: a brief review.BMB Rep. 2024 Aug;57(8):343-351. doi: 10.5483/BMBRep.2024-0082. BMB Rep. 2024. PMID: 39044455 Free PMC article. Review.
Cited by
-
Cancer-associated mesothelial cell-derived ANGPTL4 and STC1 promote the early steps of ovarian cancer metastasis.JCI Insight. 2023 Mar 22;8(6):e163019. doi: 10.1172/jci.insight.163019. JCI Insight. 2023. PMID: 36795484 Free PMC article.
-
Integrated single-cell and spatial transcriptomics reveals heterogeneity of fibroblast and pivotal genes in psoriasis.Sci Rep. 2023 Oct 10;13(1):17134. doi: 10.1038/s41598-023-44346-6. Sci Rep. 2023. PMID: 37816883 Free PMC article.
-
Emerging roles of angiopoietin‑like 4 in human tumors (Review).Int J Oncol. 2025 Feb;66(2):9. doi: 10.3892/ijo.2024.5715. Epub 2024 Dec 20. Int J Oncol. 2025. PMID: 39704206 Free PMC article. Review.
-
Predictive and prognostic models and visualizations of distant metastasis in gallbladder cancer.Medicine (Baltimore). 2025 Jul 18;104(29):e43369. doi: 10.1097/MD.0000000000043369. Medicine (Baltimore). 2025. PMID: 40696595 Free PMC article.
-
Bioinformatic analysis of cancer-associated fibroblast related gene signature as a predictive model in clinical outcomes and immune characteristics of gastric cancer.Ann Transl Med. 2022 Jun;10(12):698. doi: 10.21037/atm-22-2810. Ann Transl Med. 2022. PMID: 35845527 Free PMC article.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. Ca‐Cancer J Clin. 2016;66:7‐30. - PubMed
-
- Kim BH, Kwon J, Chie EK, et al. Adjuvant chemoradiotherapy is associated with improved survival for patients with resected gallbladder carcinoma: A systematic review and meta‐analysis. Ann Surg Oncol. 2018;25:255‐264. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 30672073/National Natural Science Foundation of China
- 81372614/National Natural Science Foundation of China
- 13ZR1432300/Shanghai Science and Technology Commission Research Project
- 19140902302/Shanghai Science and Technology Commission Research Project
- 19411966300/Shanghai Science and Technology Commission Research Project
LinkOut - more resources
Full Text Sources
Medical
Research Materials

