Clinical ApoA-IV amyloid is associated with fibrillogenic signal sequence
- PMID: 34331462
- PMCID: PMC9291309
- DOI: 10.1002/path.5770
Clinical ApoA-IV amyloid is associated with fibrillogenic signal sequence
Abstract
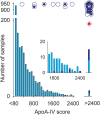
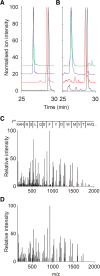
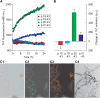
Apolipoprotein A-IV amyloidosis is an uncommon form of the disease normally resulting in renal and cardiac dysfunction. ApoA-IV amyloidosis was identified in 16 patients attending the National Amyloidosis Centre and in eight clinical samples received for histology review. Unexpectedly, proteomics identified the presence of ApoA-IV signal sequence residues (p.18-43 to p.20-43) in 16/24 trypsin-digested amyloid deposits but in only 1/266 non-ApoA-IV amyloid samples examined. These additional signal residues were also detected in the cardiac sample from the Swedish patient in which ApoA-IV amyloid was first described, and in plasma from a single cardiac ApoA-IV amyloidosis patient. The most common signal-containing peptide observed in ApoA-IV amyloid, p.20-43, and to a far lesser extent the N-terminal peptide, p.21-43, were fibrillogenic in vitro at physiological pH, generating Congo red-positive fibrils. The addition of a single signal-derived alanine residue to the N-terminus has resulted in markedly increased fibrillogenesis. If this effect translates to the mature circulating protein in vivo, then the presence of signal may result in preferential deposition as amyloid, perhaps acting as seed for the main circulating native form of the protein; it may also influence other ApoA-IV-associated pathologies. © 2021 The Authors. The Journal of Pathology published by John Wiley & Sons, Ltd. on behalf of The Pathological Society of Great Britain and Ireland.
Keywords: ApoA-IV amyloidosis; ApoA-IV sequence coverage; ApoA-IV signal-containing peptide; amyloid proteomics; fibrillogenic ApoA-IV signal-containing peptide; targeted mass spectrometry in serum.
© 2021 The Authors. The Journal of Pathology published by John Wiley & Sons, Ltd. on behalf of The Pathological Society of Great Britain and Ireland.
Figures
Similar articles
-
Amyloid-forming property of the N-terminal 1-70 residues of human apolipoprotein A-IV.Sci Rep. 2025 Apr 16;15(1):13203. doi: 10.1038/s41598-025-97992-3. Sci Rep. 2025. PMID: 40240491 Free PMC article.
-
[Classification of cardiac amyloidosis: an immunohistochemical analysis].Zhonghua Bing Li Xue Za Zhi. 2018 Feb 8;47(2):105-109. doi: 10.3760/cma.j.issn.0529-5807.2018.02.005. Zhonghua Bing Li Xue Za Zhi. 2018. PMID: 29429161 Chinese.
-
Apolipoprotein A-1-related amyloidosis 2 case reports and review of the literature.Medicine (Baltimore). 2017 Sep;96(39):e8148. doi: 10.1097/MD.0000000000008148. Medicine (Baltimore). 2017. PMID: 28953655 Free PMC article.
-
Pathology and diagnosis of renal non-AL amyloidosis.J Nephrol. 2018 Jun;31(3):343-350. doi: 10.1007/s40620-017-0426-6. Epub 2017 Aug 21. J Nephrol. 2018. PMID: 28828707 Review.
-
The crystal structure of the C-terminal truncated apolipoprotein A-I sheds new light on amyloid formation by the N-terminal fragment.Biochemistry. 2012 Jan 10;51(1):10-8. doi: 10.1021/bi2017014. Epub 2011 Dec 29. Biochemistry. 2012. PMID: 22229410 Free PMC article. Review.
Cited by
-
Small Bowel Perforation in Roux-en-Y Gastric Bypass (RYGB) Secondary to Apolipoprotein A-IV (AApoA-IV) Type Amyloidosis.Obes Surg. 2025 May;35(5):1679-1684. doi: 10.1007/s11695-025-07814-8. Epub 2025 Mar 29. Obes Surg. 2025. PMID: 40156753 Free PMC article.
-
An unusual phenotype of hereditary AApoAI amyloidosis caused by a novel Asp20Tyr substitution is linked to pH-dependent aggregation of apolipoprotein A-I.Biochim Biophys Acta Mol Basis Dis. 2025 Jun;1871(5):167820. doi: 10.1016/j.bbadis.2025.167820. Epub 2025 Mar 29. Biochim Biophys Acta Mol Basis Dis. 2025. PMID: 40164396
-
Age-related TFEB downregulation in proximal tubules causes systemic metabolic disorders and occasional apolipoprotein A4-related amyloidosis.JCI Insight. 2024 Dec 19;10(3):e184451. doi: 10.1172/jci.insight.184451. JCI Insight. 2024. PMID: 39699959 Free PMC article.
References
-
- Pepys MB. Amyloidosis. Annu Rev Med 2006; 57: 223–241. - PubMed
-
- Benson MD, Buxbaum JN, Eisenberg DS, et al. Amyloid nomenclature 2020: update and recommendations by the International Society of Amyloidosis (ISA) nomenclature committee. Amyloid 2020; 27: 217–222. - PubMed
-
- Galant NJ, Westermark P, Higaki JN, et al. Transthyretin amyloidosis: an under‐recognized neuropathy and cardiomyopathy. Clin Sci (Lond) 2017; 131: 395–409. - PubMed
-
- Falk RH, Alexander KM, Liao R, et al. AL (light‐chain) cardiac amyloidosis: a review of diagnosis and therapy. J Am Coll Cardiol 2016; 68: 1323–1341. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

