Kidney transplantation from triple-knockout pigs expressing multiple human proteins in cynomolgus macaques
- PMID: 34331749
- PMCID: PMC9291868
- DOI: 10.1111/ajt.16780
Kidney transplantation from triple-knockout pigs expressing multiple human proteins in cynomolgus macaques
Abstract
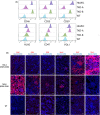
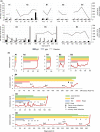
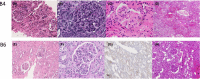
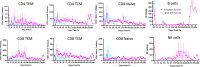
Porcine cells devoid of three major carbohydrate xenoantigens, αGal, Neu5GC, and SDa (TKO) exhibit markedly reduced binding of human natural antibodies. Therefore, it is anticipated that TKO pigs will be better donors for human xenotransplantation. However, previous studies on TKO pigs using old world monkeys (OWMs) have been disappointing because of higher anti-TKO pig antibodies in OWMs than humans. Here, we show that long-term survival of renal xenografts from TKO pigs that express additional human transgenes (hTGs) can be achieved in cynomolgus monkeys. Kidney xenografts from TKO-hTG pigs were transplanted into eight cynomolgus recipients without pre-screening for low anti-pig antibody titers. Two recipients of TKO-hTG xenografts with low expression of human complement regulatory proteins (CRPs) (TKO-A) survived for 2 and 61 days, whereas six recipients of TKO-hTG xenografts with high CRP expression (TKO-B) survived for 15, 20, 71, 135, 265, and 316 days. Prolonged CD4+ T cell depletion and low anti-pig antibody titers, which were previously reported important for long-term survival of αGal knock-out (GTKO) xenografts, were not always required for long-term survival of TKO-hTG renal xenografts. This study indicates that OWMs such as cynomolgus monkeys can be used as a relevant model for clinical application of xenotransplantation using TKO pigs.
Keywords: immunosuppression/immune modulation; translational research/science; xenoantibody; xenoantigen; xenotransplantation.
© 2021 The Authors. American Journal of Transplantation published by Wiley Periodicals LLC on behalf of The American Society of Transplantation and the American Society of Transplant Surgeons.
Conflict of interest statement
The authors of this manuscript have conflicts of interest to disclose as described by the
Figures


References
-
- UNOS Transplant trends. 2020. https://unos.org/data/transplant‐trends/. Accessed February 20, 2021.
-
- Nunes dos Santos RM, Carneiro D'Albuquerque LA, Reyes LM, et al. CRISPR/Cas and recombinase‐based human‐to‐pig orthotopic gene exchange for xenotransplantation. J Surg Res. 2018;229:28‐40. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

