A photo-cross-linking GlcNAc analog enables covalent capture of N-linked glycoprotein-binding partners on the cell surface
- PMID: 34331854
- PMCID: PMC8792112
- DOI: 10.1016/j.chembiol.2021.07.007
A photo-cross-linking GlcNAc analog enables covalent capture of N-linked glycoprotein-binding partners on the cell surface
Abstract
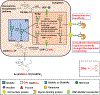
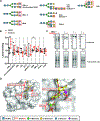
N-glycans are displayed on cell-surface proteins and can engage in direct binding interactions with membrane-bound and secreted glycan-binding proteins (GBPs). Biochemical identification and characterization of glycan-mediated interactions is often made difficult by low binding affinities. Here we describe the metabolic introduction of a diazirine photo-cross-linker onto N-acetylglucosamine (GlcNAc) residues of N-linked glycoproteins on cell surfaces. We characterize sites at which diazirine-modified GlcNAc is incorporated, as well as modest perturbations to glycan structure. We show that diazirine-modified GlcNAc can be used to covalently cross-link two extracellular GBPs, galectin-1 and cholera toxin subunit B, to cell-surface N-linked glycoproteins. The extent of cross-linking correlates with display of the preferred glycan ligands for the GBPs. In addition, covalently cross-linked complexes could be isolated, and protein components of cross-linked N-linked glycoproteins were identified by proteomics analysis. This method may be useful in the discovery and characterization of binding interactions that depend on N-glycans.
Keywords: N-glycan; cholera; cross-linking; diazirine; galectin; glycosylation; glycosyltransferase.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests K.W.M. acknowledges ownership interest and roles as president and CEO of Glyco Expression Technologies, Inc., a biotechnology spinout commercializing recombinant glycosyltransferases, and may conceivably profit from the results described herein. J.J.K. is a member of the advisory board for Cell Chemical Biology. Other authors declare no competing interests.
Figures




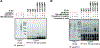
References
-
- Camby I, Le Mercier M, Lefranc F, and Kiss R (2006). Galectin-1: a small protein with major functions. Glycobiology 16, 137R–157R. - PubMed
-
- Cummings RD, and Kornfeld S (1984). The distribution of repeating [Gal beta 1,4GlcNAc beta 1,3] sequences in asparagine-linked oligosaccharides of the mouse lymphoma cell lines BW5147 and PHAR 2.1. J Biol Chem 259, 6253–6260. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

