Mini review: Biomaterials in repair and regeneration of nerve in a volumetric muscle loss
- PMID: 34332029
- PMCID: PMC8529875
- DOI: 10.1016/j.neulet.2021.136145
Mini review: Biomaterials in repair and regeneration of nerve in a volumetric muscle loss
Abstract
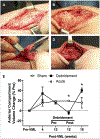
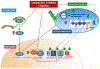
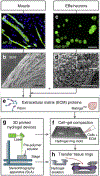
Volumetric muscle loss (VML) following a severe trauma or injury is beyond the intrinsic regenerative capacity of muscle tissues, and hence interventional therapy is required. Extensive muscle loss concomitant with damage to neuromuscular components overwhelms the muscles' remarkable regenerative capacity. The loss of nervous and vascular tissue leads to further damage and atrophy, so a combined treatment for neuromuscular junction (NMJ) along with the volumetric muscle regeneration is important. There have been immense advances in the field of tissue engineering for skeletal muscle tissue and peripheral nerve regeneration, but very few address the interdependence of the tissues and the need for combined therapies to repair and regenerate fully functional muscle tissue. This review addresses the problem and presents an overview of the biomaterials that have been studied for tissue engineering of neuromuscular tissues associated with skeletal muscles.
Keywords: Muscle regeneration; Nerve regeneration; Neuromuscular junction; Tissue engineering.
Copyright © 2021 Elsevier B.V. All rights reserved.
Figures




Similar articles
-
Neuromuscular Regeneration of Volumetric Muscle Loss Injury in Response to Agrin-Functionalized Tissue Engineered Muscle Grafts and Rehabilitative Exercise.Adv Healthc Mater. 2025 Jan;14(2):e2403028. doi: 10.1002/adhm.202403028. Epub 2024 Nov 10. Adv Healthc Mater. 2025. PMID: 39523723
-
Differential evaluation of neuromuscular injuries to understand re-innervation at the neuromuscular junction.Exp Neurol. 2024 Dec;382:114996. doi: 10.1016/j.expneurol.2024.114996. Epub 2024 Oct 10. Exp Neurol. 2024. PMID: 39393669
-
Vascularized and Innervated Skeletal Muscle Tissue Engineering.Adv Healthc Mater. 2020 Jan;9(1):e1900626. doi: 10.1002/adhm.201900626. Epub 2019 Oct 17. Adv Healthc Mater. 2020. PMID: 31622051 Free PMC article. Review.
-
Naturally derived and synthetic scaffolds for skeletal muscle reconstruction.Adv Drug Deliv Rev. 2015 Apr;84:208-21. doi: 10.1016/j.addr.2014.08.011. Epub 2014 Aug 29. Adv Drug Deliv Rev. 2015. PMID: 25174309 Free PMC article. Review.
-
Pre-innervated tissue-engineered muscle promotes a pro-regenerative microenvironment following volumetric muscle loss.Commun Biol. 2020 Jun 25;3(1):330. doi: 10.1038/s42003-020-1056-4. Commun Biol. 2020. PMID: 32587337 Free PMC article.
Cited by
-
An electroencephalography-based human-machine interface combined with contralateral C7 transfer in the treatment of brachial plexus injury.Neural Regen Res. 2022 Dec;17(12):2600-2605. doi: 10.4103/1673-5374.335838. Neural Regen Res. 2022. PMID: 35662188 Free PMC article. Review.
-
Magnetic Nanofibrous Scaffolds Accelerate the Regeneration of Muscle Tissue in Combination with Extra Magnetic Fields.Int J Mol Sci. 2022 Apr 18;23(8):4440. doi: 10.3390/ijms23084440. Int J Mol Sci. 2022. PMID: 35457258 Free PMC article.
-
Accelerated innervation of biofabricated skeletal muscle implants containing a neurotrophic factor delivery system.Front Bioeng Biotechnol. 2024 Oct 28;12:1476370. doi: 10.3389/fbioe.2024.1476370. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39530055 Free PMC article.
-
Advancements in skeletal muscle tissue engineering: strategies for repair and regeneration of skeletal muscle beyond self-repair.Regen Biomater. 2025 May 28;12:rbaf050. doi: 10.1093/rb/rbaf050. eCollection 2025. Regen Biomater. 2025. PMID: 40599408 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

