A glypican-1-targeted antibody-drug conjugate exhibits potent tumor growth inhibition in glypican-1-positive pancreatic cancer and esophageal squamous cell carcinoma
- PMID: 34332450
- PMCID: PMC8340053
- DOI: 10.1016/j.neo.2021.07.006
A glypican-1-targeted antibody-drug conjugate exhibits potent tumor growth inhibition in glypican-1-positive pancreatic cancer and esophageal squamous cell carcinoma
Abstract
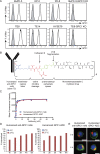
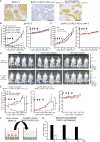
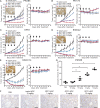
An antibody-drug conjugate (ADC) is a promising therapeutic modality because selective and effective delivery of an anti-cancer drug is achieved by drug-conjugated antibody-targeting cancer antigen. Glypican 1 (GPC1) is highly expressed in malignant tumors, including pancreatic ductal adenocarcinoma (PDAC) and esophageal squamous cell carcinoma (ESCC). Herein, we describe the usefulness of GPC1-targeting ADC. Humanized anti-GPC1 antibody (clone T2) was developed and conjugated with monomethyl auristatin E (MMAE) via maleimidocaproyl-valine-citrulline-p-aminobenzyloxycarbonyl (mc-vc-PABC) linkers (humanized GPC1-ADC[MMAE]). Humanized GPC1-ADC(MMAE) inhibited the growth of GPC1-positive PDAC and ESCC cell lines via inducing cycle arrest in the G2/M phase and apoptosis in vitro. The binding activity of humanized GPC1-ADC(MMAE) with GPC1 was comparable with that of the unconjugated anti-GPC1 antibody. The humanized GPC1-ADC(MMAE) was effective in GPC1-positive BxPC-3 subcutaneously xenografted mice but not in GPC1-negative BxPC-3-GPC1-KO xenografted mice. To assess the bystander killing activity of the humanized GPC1-ADC(MMAE), a mixture of GPC1-positive BxPC-3 and GPC1-negative BxPC-3-GPC1-KO-Luc cells were subcutaneously inoculated, and a heterogenous GPC1-expressing tumor model was developed. The humanized GPC1-ADC(MMAE) inhibited the tumor growth and decreased the luciferase signal, measured with an in vivo imaging system (IVIS), which suggests that the suppression of the BxPC-3-GPC1-KO-Luc population. The humanized GPC1-ADC(MMAE) also inhibited the established liver metastases of BxPC-3 cells and significantly improved the overall survival of the mice. It exhibited a potent antitumor effect on the GPC1-positive PDAC and ESCC patient-derived xenograft (PDX) models. Our preclinical data demonstrate that GPC1 is a promising therapeutic target for ADC.
Keywords: Antibody-drug conjugate; Esophageal squamous cell carcinoma; Glypican-1; Pancreatic cancer.
Copyright © 2021. Published by Elsevier Inc.
Figures


References
-
- Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. - PubMed
-
- Uhlenhopp DJ, Then EO, Sunkara T, Gaduputi V. Epidemiology of esophageal cancer: update in global trends, etiology and risk factors. Clin J Gastroenterol. 2020;6:1010–1021. - PubMed
-
- Katz J, Janik JE, Younes A. Brentuximab Vedotin (SGN-35) Clin Cancer Res. 2011;17:6428–6436. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

