Lung donation following SARS-CoV-2 infection
- PMID: 34332512
- PMCID: PMC8441925
- DOI: 10.1111/ajt.16777
Lung donation following SARS-CoV-2 infection
Abstract
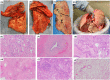
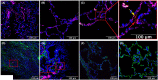
There have been over 177 million cases of COVID-19 worldwide, many of whom could be organ donors. Concomitantly, there is an anticipated increase in the need for donor lungs due to expanding indications. Given that the respiratory tract is most commonly affected by COVID-19, there is an urgent need to develop donor assessment criteria while demonstrating safety and "efficacy" of lung donation following COVID-19 infection. Accordingly, we report an intentional transplant using lungs from a donor with recent, microbiologically confirmed, COVID-19 infection into a recipient suffering from COVID-19 induced ARDS and pulmonary fibrosis. In addition to the standard clinical assays, both donor and recipient lungs were analyzed using RNAscope, which confirmed that tissues were negative for SARS-CoV-2. Immunohistochemistry demonstrated colocalized KRT17+ basaloid-like epithelium and COL1A1+ fibroblasts, a marker suggestive of lung fibrosis in COVID-19 associated lung disease, in the explanted recipient lungs but absent in the donor lungs. We demonstrate that following a thorough assessment, lung donation following resolved COVID-19 infection is safe and feasible.
Keywords: clinical research/practice; donors and donation; lung transplantation/pulmonology; lung transplantation: living donor.
© 2021 The American Society of Transplantation and the American Society of Transplant Surgeons.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

