Association between inflammatory markers and serum paraoxonase and arylesterase activities in the general population: a cross-sectional study
- PMID: 34332593
- PMCID: PMC8325814
- DOI: 10.1186/s12944-021-01508-7
Association between inflammatory markers and serum paraoxonase and arylesterase activities in the general population: a cross-sectional study
Abstract
Background: Recent studies focused on modulating factors of paraoxonase-1 (PON1) activity. In some studies the association between pro-inflammatory markers and PON1 activity was examined, but so far no population-based investigations on this issue have been conducted. The present study investigated the relationships between the pro-inflammatory markers tumor necrosis factor (TNF)-α, leptin, interleukin (IL)-6, and high-sensitive C-reactive protein (hs-CRP) and paraoxonase and arylesterase, two hydrolytic activities of PON1, in the population-based Bavarian Food Consumption Survey II.
Methods: Based on 504 participants (217 men, 287 women), the relationship between the pro-inflammatory markers and the outcomes paraoxonase and arylesterase activities were investigated using multivariable linear models.
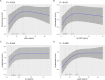
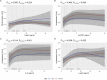
Results: Circulating plasma levels of leptin (P-value < 0.0001), hs-CRP (P-value = 0.031) and IL-6 (P-value = 0.045) were significantly non-linearly associated with arylesterase activity. Leptin levels were also significantly associated with paraoxonase activity (P-value = 0.024) independently from confounding factors, including high-density lipoprotein (HDL) cholesterol. With increasing levels of these inflammatory parameters, arylesterase and paraoxonase activities increased; however, at higher levels (> 75th percentile) the activities reached a plateau or even decreased somewhat. After Bonferroni-Holm correction, only leptin remained non-linearly but significantly associated with arylesterase activity (adjusted overall P-value < 0.0001). Neither age nor sex nor obesity modified the associations. No association was found between TNF-α and paraoxonase or arylesterase activity.
Conclusions: The present findings suggest that in persons with very high levels of inflammation, PON1 activity may be impaired, a fact that might subsequently be accompanied by a higher risk for cardiometabolic diseases. Whether or not the measurement of PON1 activity in combination with a lipid profile and certain inflammatory markers could improve the prediction of cardiometabolic diseases in middle-aged individuals from the general population should be evaluated in clinical studies.
Keywords: Arylesterase activity; Inflammation; Oxidative stress; PON1; Paraoxonase activity.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Association between alcohol consumption and serum paraoxonase and arylesterase activities: a cross-sectional study within the Bavarian population.Br J Nutr. 2016 Feb 28;115(4):730-6. doi: 10.1017/S0007114515004985. Epub 2016 Jan 15. Br J Nutr. 2016. PMID: 26769660
-
Paraoxonase, arylesterase and lactonase activities of paraoxonase-1 (PON1) in obese and severely obese women.Scand J Clin Lab Invest. 2018 Feb-Apr;78(1-2):18-24. doi: 10.1080/00365513.2017.1405274. Epub 2017 Nov 23. Scand J Clin Lab Invest. 2018. PMID: 29168398
-
Serum paraoxonase-1 activity in Helicobacter pylori infected subjects.Atherosclerosis. 2008 Jan;196(1):270-274. doi: 10.1016/j.atherosclerosis.2006.10.024. Epub 2006 Nov 27. Atherosclerosis. 2008. PMID: 17125774
-
Paraoxonase 1 gene polymorphisms in lipid oxidation and atherosclerosis development.Front Genet. 2022 Sep 2;13:966413. doi: 10.3389/fgene.2022.966413. eCollection 2022. Front Genet. 2022. PMID: 36118876 Free PMC article. Review.
-
Paraoxonase/Arylesterase Activity of Serum Paraoxonase-1 and Schizophrenia: A Systematic Review and Meta-Analysis.Antioxidants (Basel). 2023 Jul 25;12(8):1484. doi: 10.3390/antiox12081484. Antioxidants (Basel). 2023. PMID: 37627479 Free PMC article. Review.
Cited by
-
The Complexity of Oxidative Stress in Human Age-Related Diseases-A Review.Metabolites. 2025 Jul 15;15(7):479. doi: 10.3390/metabo15070479. Metabolites. 2025. PMID: 40710578 Free PMC article. Review.
-
Importance of Polymorphisms in the Gene of Paraoxonase-1 (SNP rs662) and Apolipoprotein A-I (SNP rs670 and rs5069) in Non-Smoking and Smoking Healthy Subjects and Patients with Acute Pancreatitis.Genes (Basel). 2022 Oct 28;13(11):1968. doi: 10.3390/genes13111968. Genes (Basel). 2022. PMID: 36360205 Free PMC article.
-
Paraoxonase-1 as a Cardiovascular Biomarker in Caribbean Hispanic Patients Treated with Clopidogrel: Abundance and Functionality.Int J Mol Sci. 2024 Oct 3;25(19):10657. doi: 10.3390/ijms251910657. Int J Mol Sci. 2024. PMID: 39408985 Free PMC article.
-
PON-1 and PON-2 Polymorphisms and PON-1 Paraoxonase Activity in People Living with HIV-1.Antioxidants (Basel). 2025 Feb 12;14(2):209. doi: 10.3390/antiox14020209. Antioxidants (Basel). 2025. PMID: 40002395 Free PMC article.
-
A systematic review and meta-analysis of paraoxonase-1 activity in asthma.Clin Exp Med. 2023 Aug;23(4):1067-1074. doi: 10.1007/s10238-022-00930-0. Epub 2022 Nov 7. Clin Exp Med. 2023. PMID: 36344783 Free PMC article.
References
-
- Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145(5):2273–2282. doi: 10.1210/en.2003-1336. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

