Directional homing of glycosylation-modified bone marrow mesenchymal stem cells for bone defect repair
- PMID: 34332597
- PMCID: PMC8325817
- DOI: 10.1186/s12951-021-00969-3
Directional homing of glycosylation-modified bone marrow mesenchymal stem cells for bone defect repair
Abstract
Background: One of the greatest challenges for tissue-engineered bone is the low survival rate of locally grafted cells. The cell homing technology can effectively increase the number of these grafted cells, therefore, enhancing the repair of bone defects. Here we explore the effect of fucosylation modification on the directional homing of bone marrow mesenchymal stem cells (BMSCs) and their ability to repair bone defects.
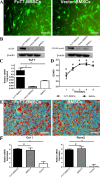
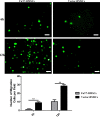
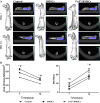
Results: Glycosylated BMSCs expressed high levels of the Sialyl Lewis-X (sLeX) antigen, which enabled the cells to efficiently bind to E- and P-selectins and to home to bone defect sites in vivo. Micro-CT and histological staining results confirmed that mice injected with FuT7-BMSCs showed an improved repair of bone defects compared to unmodified BMSCs.
Conclusions: The glycosylation modification of BMSCs has significantly enhanced their directional homing ability to bone defect sites, therefore, promoting bone repair. Our results suggest that glycosylation-modified BMSCs can be used as the source of the cells for the tissue-engineered bone and provide a new approach for the treatment of bone defects.
Keywords: Bone defect repair; Bone marrow mesenchymal stem cells; Directional homing; Glycosylation.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

