Prevention of chemotherapy-induced premature ovarian insufficiency in mice by scaffold-based local delivery of human embryonic stem cell-derived mesenchymal progenitor cells
- PMID: 34332643
- PMCID: PMC8325282
- DOI: 10.1186/s13287-021-02479-3
Prevention of chemotherapy-induced premature ovarian insufficiency in mice by scaffold-based local delivery of human embryonic stem cell-derived mesenchymal progenitor cells
Abstract
Background: Premature ovarian insufficiency (POI) is one of the most serious side effects of chemotherapy in young cancer survivors. It may not only reduce fecundity but also affect lifelong health. There is no standard therapy for preserving ovarian health after chemotherapy. Recently, administration of embryonic stem cell-derived mesenchymal progenitor cells (ESC-MPCs) has been considered a new therapeutic option for preventing POI. However, the previous method of directly injecting cells into the veins of patients exhibits low efficacy and safety. This study aimed to develop safe and effective local delivery methods for the prevention of POI using two types of bioinspired scaffolds.
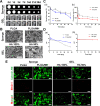
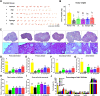
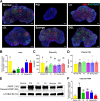
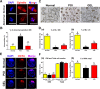
Methods: Female mice received intraperitoneal cisplatin for 10 days. On day 11, human ESC-MPCs were delivered through systemic administration using intravenous injection or local administration using intradermal injection and intradermal transplantation with a PLGA/MH sponge or hyaluronic acid (HA) gel (GEL) type of scaffold. PBS was injected intravenously as a negative control. Ovarian function and fertility were evaluated 4 weeks after transplantation. Follicle development was observed using hematoxylin and eosin staining. The plasma levels of sex hormones were measured using ELISA. Expression levels of anti-Müllerian hormone (AMH) and ki-67 were detected using immunostaining, and the quality of oocytes and embryos was evaluated after in vitro fertilization. The estrous cycles were observed at 2 months after transplantation.
Results: The local administration of human ESC-MPCs using the bioinspired scaffold to the backs of mice effectively prolonged the cell survival rate in vivo. The HA GEL group exhibited the best recovered ovarian functions, including a significantly increased number of ovarian reserves, estrogen levels, and AMH levels and decreased apoptotic levels. Furthermore, the HA GEL group showed improved quality of oocytes and embryos and estrous cycle regularity.
Conclusions: HA GEL scaffolds can be used as new delivery platforms for ESC-MPC therapy, and this method may provide a novel option for the clinical treatment of chemotherapy-induced POI.
Keywords: Bioinspired scaffold; Cell therapy; Chemotherapy-induced premature ovarian insufficiency; Embryonic stem cell-derived mesenchymal progenitor cells; Hyaluronic acid; Local delivery; Oncofertility.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Koyama H, Wada T, Nishizawa Y, Iwanaga T, Aoki Y, Terasawa T, Kosaki G, Yamamoto T, Wada A. Cyclophosphamide-induced ovarian failure and its therapeutic significance in patients with breast cancer. Cancer. 1977;39(4):1403–1409. doi: 10.1002/1097-0142(197704)39:4<1403::AID-CNCR2820390408>3.0.CO;2-8. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

