Determination of Cutpoints for Symptom Burden in Oncology Patients Receiving Chemotherapy
- PMID: 34333099
- PMCID: PMC10791137
- DOI: 10.1016/j.jpainsymman.2021.07.018
Determination of Cutpoints for Symptom Burden in Oncology Patients Receiving Chemotherapy
Abstract
Context: Cutpoints can be used as a threshold for screening symptom(s) that warrant intervention(s) and for monitoring patients' responses to these interventions.
Objectives: In a sample of oncology patients undergoing chemotherapy, study purposes were to determine the optimal cutpoints for low, moderate, and high symptom burden and determine if these cutpoints distinguished among the symptom groups in any demographic, clinical, and stress characteristics, as well as QOL outcomes.
Methods: Total of 1329 patients completed a modified version of the Memorial Symptom Assessment Scale (38 symptoms). Using the methodology of Serlin and colleagues, cutpoints were created using symptom occurrence rates and cancer-specific quality of life (QOL) scores. Cutpoints were validated using measures of stress and resilience and a generic measure of QOL (i.e., Medical Outcomes Study Short Form 12 (SF-12)).
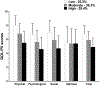
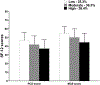
Results: Of the 25 possible cutpoints evaluated, the optimal cutpoint, with the largest between category F statistic, was CP8,15 (Low = 0-8, Moderate = 9-15, High = 16-38 symptoms). Percentage of patients in the Low, Moderate, and High cutpoint groups were 25.3%, 36.3%, and 38.4%, respectively. Significant differences were found among the symptom burden groups in global, cancer-specific, and cumulative life stress (i.e., Low < Moderate < High) and resilience and SF-12 (i.e., Low > Moderate > High) scores.
Conclusion: Our findings provide evidence for clinically meaningful cutpoints that can be used to guide symptom assessment and management. These cutpoints may be used to establish alert thresholds for electronic monitoring of symptoms in oncology patients.
Keywords: Symptom burden; cancer; chemotherapy; cutpoints; severity; symptoms.
Copyright © 2021 American Academy of Hospice and Palliative Medicine. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interest: The authors have no conflicts of interest to declare.
Figures
References
-
- Shi Q, Mendoza TR, Cleeland CS. Interpreting patient-reported outcome scores for clinical research and practice: Definition, determination, and application of cutpoints. Med Care 2019;57 Suppl 5 Suppl 1:S8–S12. - PubMed
-
- Paul SM, Zelman DC, Smith M, Miaskowski C. Categorizing the severity of cancer pain: further exploration of the establishment of cutpoints. Pain 2005;113:37–44. - PubMed
-
- Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain 1995;61:277–284. - PubMed
-
- Chang YJ, Lee JS, Lee CG, et al. Assessment of clinical relevant fatigue level in cancer. Support Care Cancer 2007;15:891–896. - PubMed

