Protease inhibitor-based direct-acting antivirals are associated with increased risk of aminotransferase elevations but not hepatic dysfunction or decompensation
- PMID: 34333102
- PMCID: PMC8604762
- DOI: 10.1016/j.jhep.2021.07.021
Protease inhibitor-based direct-acting antivirals are associated with increased risk of aminotransferase elevations but not hepatic dysfunction or decompensation
Abstract
Background & aims: Cases of acute liver injury (ALI) have been reported among chronic HCV-infected patients receiving protease inhibitor (PI)-based direct-acting antiviral (DAA) regimens, but no analyses have compared the risk of ALI in patients receiving PI- vs. non-PI-based DAAs. Thus, we compared the risk of 3 ALI outcomes between patients (by baseline Fibrosis-4 [FIB-4] group) receiving PI-based or non-PI-based DAAs.
Methods: We conducted a cohort study of 18,498 patients receiving PI-based DAA therapy (paritaprevir/ritonavir/ombitasvir±dasabuvir, elbasvir/grazoprevir, glecaprevir/pibrentasvir) matched 1:1 on propensity score to those receiving non-PI-based DAAs (sofosbuvir/ledipasvir, sofosbuvir/velpatasvir) in the 1945-1965 Veterans Birth Cohort (2014-2019). During exposure to DAA therapy, we determined development of: i) alanine aminotransferase (ALT) >200 U/L, ii) severe hepatic dysfunction (coagulopathy with hyperbilirubinemia), and iii) hepatic decompensation. We used Cox regression to determine hazard ratios (HRs) with 95% CIs for each ALI outcome within groups defined by baseline FIB-4 (≤3.25; >3.25).
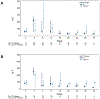
Results: Among patients with baseline FIB-4 ≤3.25, those receiving PIs had a higher risk of ALT >200 U/L (HR 3.98; 95% CI 2.37-6.68), but not severe hepatic dysfunction (HR 0.67; 95% CI 0.19-2.39) or hepatic decompensation (HR 1.01; 95% CI 0.29-3.49), compared to those receiving non-PI-based regimens. For those with baseline FIB-4 >3.25, those receiving PIs had a higher risk of ALT >200 U/L (HR, 2.15; 95% CI 1.09-4.26), but not severe hepatic dysfunction (HR, 1.23 [0.64-2.38]) or hepatic decompensation (HR, 0.87; 95% CI 0.41-1.87), compared to those receiving non-PI-based regimens CONCLUSION: While risk of incident ALT elevations was increased in those receiving PI-based DAAs in both FIB-4 groups, the risk of severe hepatic dysfunction and hepatic decompensation did not differ between patients receiving PI- or non-PI-based DAAs in either FIB-4 group.
Lay summary: Cases of liver injury have been reported among patients treated with protease inhibitor-based direct-acting antivirals for hepatitis C infection, but it is not clear if the risk of liver injury among people starting these drugs is increased compared to those starting non-protease inhibitor-based therapy. In this study, patients receiving protease inhibitor-based treatment had a higher risk of liver inflammation than those receiving a non-protease inhibitor-based treatment, regardless of the presence of pre-treatment advanced liver fibrosis/cirrhosis. However, the risk of severe liver dysfunction and decompensation were not higher for patients treated with protease inhibitor-based regimens.
Keywords: acute liver injury; direct-acting antivirals; hepatitis C; protease inhibitor.
Published by Elsevier B.V.
Conflict of interest statement
Conflict of interest D.B. has received research support to the NIH AIDS Clinical Trials Group (ACTG) from Abbvie and Regeneron for an ACTG-sponsored trial. All other authors declare no conflicts of interest. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures

Comment in
-
Is it safe to treat chronic hepatitis C patients with decompensated cirrhosis with PI-based DAAs?J Hepatol. 2022 Jul;77(1):257-258. doi: 10.1016/j.jhep.2021.12.037. Epub 2022 Jan 21. J Hepatol. 2022. PMID: 35074472 No abstract available.
-
Reply to: "Is it safe to treat chronic hepatitis C patients with decompensated cirrhosis with PI-based DAAs?".J Hepatol. 2022 Jul;77(1):258-259. doi: 10.1016/j.jhep.2022.02.025. Epub 2022 Mar 10. J Hepatol. 2022. PMID: 35283213 No abstract available.
References
-
- United States Food and Drug Administration. FDA Drug Safety Communication: FDA warns of serious liver injury risk with hepatitis C treatments Viekira Pak and Technivie. Oct 22, 2015. Accessed at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-c.... Accessed on March 28, 2021.
-
- United States Food and Drug Administration. FDA Drug Safety Communication: FDA warns about rare occurrence of serious liver injury with use of hepatitis C medicines Mavyret, Zepatier, and Vosevi in some patients with advanced liver disease. Accessed at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-about-r.... Accessed on March 28, 2021.
-
- Mak LY, Seto WK, Lai CL, Yuen MF. An update on the toxicological considerations for protease inhibitors used for hepatitis C infection. Expert Opin Drug Metab Toxicol 2018; 14(5): 483–91. - PubMed
-
- American Association for the Study of Liver Diseases/Infectious Diseases Society of America. HCV Guidance: Recommendations for testing m, and treating hepatitis C. Accessed at: https://www.hcvguidelines.org. Accessed on March 28, 2021.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

