The cyto-protective effects of LH on ovarian reserve and female fertility during exposure to gonadotoxic alkylating agents in an adult mouse model
- PMID: 34333622
- PMCID: PMC8373474
- DOI: 10.1093/humrep/deab165
The cyto-protective effects of LH on ovarian reserve and female fertility during exposure to gonadotoxic alkylating agents in an adult mouse model
Abstract
Study question: Does LH protect mouse oocytes and female fertility from alkylating chemotherapy?
Summary answer: LH treatment before and during chemotherapy prevents detrimental effects on follicles and reproductive lifespan.
What is known already: Chemotherapies can damage the ovary, resulting in premature ovarian failure and reduced fertility in cancer survivors. LH was recently suggested to protect prepubertal mouse follicles from chemotoxic effects of cisplatin treatment.
Study design, size, duration: This experimental study investigated LH effects on primordial follicles exposed to chemotherapy. Seven-week-old CD-1 female mice were randomly allocated to four experimental groups: Control (n = 13), chemotherapy (ChT, n = 15), ChT+LH-1x (n = 15), and ChT+LH-5x (n = 8). To induce primary ovarian insufficiency (POI), animals in the ChT and ChT+LH groups were intraperitoneally injected with 120 mg/kg of cyclophosphamide and 12 mg/kg of busulfan, while control mice received vehicle. For LH treatment, the ChT+LH-1x and ChT+LH-5x animals received a 1 or 5 IU LH dose, respectively, before chemotherapy, then a second LH injection administered with chemotherapy 24 h later. Then, two animals/group were euthanized at 12 and 24 h to investigate the early ovarian response to LH, while remaining mice were housed for 30 days to evaluate short- and long-term reproductive outcomes. The effects of LH and chemotherapy on growing-stage follicles were analyzed in a parallel experiment. Seven-week-old NOD-SCID female mice were allocated to control (n = 5), ChT (n = 5), and ChT+LH-1x (n = 6) groups. Animals were treated as described above, but maintained for 7 days before reproductive assessment.
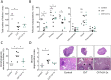
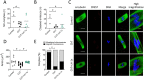
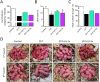
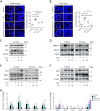
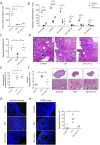
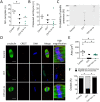
Participants/materials, setting, methods: In the first experiment, follicular damage (phosphorylated H2AX histone (γH2AX) staining and terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay), apoptotic biomarkers (western blot), and DNA repair pathways (western blot and RT-qPCR) were assessed in ovaries collected at 12 and 24 h to determine early ovarian responses to LH. Thirty days after treatments, remaining mice were stimulated (10 IU of pregnant mare serum gonadotropin (PMSG) and 10 IU of hCG) and mated to collect ovaries, oocytes, and embryos. Histological analysis was performed on ovarian samples to investigate follicular populations and stromal status, and meiotic spindle and chromosome alignment was measured in oocytes by confocal microscopy. Long-term effects were monitored by assessing pregnancy rate and litter size during six consecutive breeding attempts. In the second experiment, mice were stimulated and mated 7 days after treatments and ovaries, oocytes, and embryos were collected. Follicular numbers, follicular protection (DNA damage and apoptosis by H2AX staining and TUNEL assay, respectively), and ovarian stroma were assessed. Oocyte quality was determined by confocal analysis.
Main results and the role of chance: LH treatment was sufficient to preserve ovarian reserve and follicular development, avoid atresia, and restore ovulation and meiotic spindle configuration in mature oocytes exposed at the primordial stage. LH improved the cumulative pregnancy rate and litter size in six consecutive breeding rounds, confirming the potential of LH treatment to preserve fertility. This protective effect appeared to be mediated by an enhanced early DNA repair response, via homologous recombination, and generation of anti-apoptotic signals in the ovary a few hours after injury with chemotherapy. This response ameliorated the chemotherapy-induced increase in DNA-damaged oocytes and apoptotic granulosa cells. LH treatment also protected growing follicles from chemotherapy. LH reversed the chemotherapy-induced depletion of primordial and primary follicular subpopulations, reduced oocyte DNA damage and granulosa cell apoptosis, restored mature oocyte cohort size, and improved meiotic spindle properties.
Large scale data: N/A.
Limitations, reasons for caution: This was a preliminary study performed with mouse ovarian samples. Therefore, preclinical research with human samples is required for validation.
Wider implications of the findings: The current study tested if LH could protect the adult mouse ovarian reserve and reproductive lifespan from alkylating chemotherapy. These findings highlight the therapeutic potential of LH as a complementary non-surgical strategy for preserving fertility in female cancer patients.
Study funding/competing interest(s): This study was supported by grants from the Regional Valencian Ministry of Education (PROMETEO/2018/137), the Spanish Ministry of Science and Innovation (CP19/00141), and the Spanish Ministry of Education, Culture and Sports (FPU16/05264). The authors declare no conflict of interest.
Keywords: DNA repair; LH; cancer; chemotherapy; fertility preservation; follicle protection; ovoprotection.
© The Author(s) 2021. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Figures
References
-
- Bildik G, Akin N, Senbabaoglu F, Sahin GN, Karahuseyinoglu S, Ince U, Taskiran C, Selek U, Yakin K, Guzel Y.. et al. GnRH agonist leuprolide acetate does not confer any protection against ovarian damage induced by chemotherapy and radiation in vitro. Hum Reprod 2015;30:2912–2925. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

