Study to Compare Capsule and Liquid Formulations of Enzalutamide After Single-Dose Administration Under Fasting Conditions in Prostate Cancer
- PMID: 34333820
- PMCID: PMC8417857
- DOI: 10.1002/onco.13919
Study to Compare Capsule and Liquid Formulations of Enzalutamide After Single-Dose Administration Under Fasting Conditions in Prostate Cancer
Abstract
Lessons learned: Limited evidence suggests an acceptable pharmacokinetic profile when enzalutamide is administered via a liquid formulation extracted from the commercially available liquid-filled soft-gelatin capsules. Tolerability may limit use in clinical practice.
Background: Enzalutamide is an established standard-of-care treatment for advanced prostate cancer with a commercially available formulation that may be inconvenient for some patients. We proposed a study to evaluate the bioequivalence of a liquid formulation to provide an alternative method of administration.
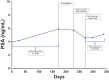
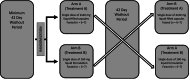
Methods: This was a single-dose, randomized, open-label, two-way crossover pilot bioequivalence study to compare two oral formulations of enzalutamide: four enzalutamide 40 mg liquid-filled soft-gelatin capsules (commercially available) administered whole versus enzalutamide 160 mg liquid (extracted from capsules) administered via oral syringe. To assess bioequivalence, patients were randomized to receive a single dose of one formulation, then cross over to receive the alternative formulation following a 42-day washout period; serial plasma samples were collected over the course of 24 hours, followed by collections at 3, 8, and 42 days after the dose for both formulations. Bioequivalence of the formulations was assessed via comparisons of area under the plasma concentration-time curve (AUC) calculations per U.S. Food and Drug Administration (FDA) guidance. The study also assessed the safety and tolerability of the formulations.
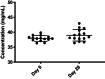
Results: The study failed to meet proposed accrual, with only one patient enrolled, thus limiting the bioequivalence evaluation. Based on the data from a single patient, the drug exposure (measured by AUC) of enzalutamide and N-desmethyl enzalutamide (primary active metabolite) for the liquid formulation was 112% and 117%, respectively, compared with the capsule formulation. Although both formulations appeared well tolerated with no adverse events reported, the tolerability assessment questionnaire revealed an unpleasant taste of the liquid formulation.
Conclusion: Preliminary evidence suggests a similar pharmacokinetic profile when administering liquid extracted from enzalutamide soft-gelatin capsules compared with intact capsules in patients with prostate cancer.
Trial registration: ClinicalTrials.gov NCT03478904.
Keywords: Bioequivalence; Enzalutamide; Liquid formulation; Pharmacokinetics; Prostate cancer.
© Published 2021. This article is a U.S. Government work and is in the public domain in the USA.
Figures

References
-
- Zelefsky MJ, Eastham JA, Cronin AM et al. Metastasis after radical prostatectomy or external beam radiotherapy for patients with clinically localized prostate cancer: A comparison of clinical cohorts adjusted for case mix. J Clin Oncol 2010;28:1508–1513. doi:10.1200/JCO.2009.22.2265 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

