Incidence comparison of adverse events in patients with inflammatory bowel disease receiving different biologic agents: retrospective long-term evaluation
- PMID: 34333908
- PMCID: PMC8831779
- DOI: 10.5217/ir.2021.00037
Incidence comparison of adverse events in patients with inflammatory bowel disease receiving different biologic agents: retrospective long-term evaluation
Abstract
Background/aims: Current literature is lacking in studies comparing the incidence of adverse events (AEs) in patients with inflammatory bowel diseases (IBD) treated with adalimumab (ADA) or vedolizumab (VDZ) in a real-life scenario. Therefore, our primary aim was to compare the AEs occurring in patients taking ADA to those of patients taking VDZ.
Methods: In this single center study, data on AEs from IBD patients who underwent treatment with ADA and VDZ were retrospectively collected. AE rates per 100 person-years were calculated. A Cox regression model was used to estimate the hazard ratios of the AEs between the 2 drugs.
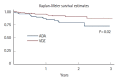
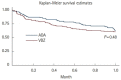
Results: A total of 16 ADA patients (17.2%) and 11 VDZ patients (7.6%) had AEs causing drug interruption during the study period (P=0.02). Most of the AEs were noninfectious extraintestinal events (50% in ADA and 54.5% in VDZ) while infections accounted for 31.2% of the AEs in patients treated with ADA and 27.3% in those treated with VDZ. The incidence rate of AEs causing withdrawal of therapy was 13.2 per 100 person-years for ADA and 5.3 per 100 person-years for VDZ, corresponding to a 76% lower risk in patients in VDZ. Considering the first year of treatment, we observed 34 subjects treated with ADA (36.5%) having at least 1 AEs and 57 (39.3%) among those taking VDZ (P=0.67).
Conclusions: VDZ has a lower incidence rate of AEs causing withdrawal of treatment compared to ADA but a similar risk of AEs not causing drug interruption. Real-life head-to-head studies are still necessary to further explore the safety profile of these drugs.
Keywords: Adalimumab; Adverse events; Biological therapy; Inflammatory bowel disease; Vedolizumab.
Conflict of interest statement
Savarino EV has received lecture or consultancy fees from Takeda, Merck & Co, Bristol-Myers Squibb, AbbVie, Amgen, Novartis, Fresenius Kabi, Sandoz, Sofar, Janssen. Card T was previously married to a subsequent employee of Takeda. Zingone F has received lecture fees from Takeda, Janssen, Norgine. The other authors declare that they have no conflicting interests.
Figures
References
-
- Maaser C, Sturm A, Vavricka SR, et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13:144–164. - PubMed
-
- Pineton de Chambrun G, Blanc P, Peyrin-Biroulet L. Current evidence supporting mucosal healing and deep remission as important treatment goals for inflammatory bowel disease. Expert Rev Gastroenterol Hepatol. 2016;10:915–927. - PubMed
-
- Torres J, Bonovas S, Doherty G, et al. ECCO guidelines on therapeutics in Crohn’s disease: medical treatment. J Crohns Colitis. 2020;14:4–22. - PubMed
-
- Barberio B, Black CJ, Savarino EV, Ford AC. Ciclosporin or infliximab as rescue therapy in acute glucorticosteroid-refractory ulcerative colitis: systematic review and network meta-analysis. J Crohns Colitis. 2021;15:733–741. - PubMed
-
- Dignass A, Lindsay JO, Sturm A, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohns Colitis. 2012;6:991–1030. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

