Early detection of COVID-19 in the UK using self-reported symptoms: a large-scale, prospective, epidemiological surveillance study
- PMID: 34334333
- PMCID: PMC8321433
- DOI: 10.1016/S2589-7500(21)00131-X
Early detection of COVID-19 in the UK using self-reported symptoms: a large-scale, prospective, epidemiological surveillance study
Abstract
Background: Self-reported symptoms during the COVID-19 pandemic have been used to train artificial intelligence models to identify possible infection foci. To date, these models have only considered the culmination or peak of symptoms, which is not suitable for the early detection of infection. We aimed to estimate the probability of an individual being infected with SARS-CoV-2 on the basis of early self-reported symptoms to enable timely self-isolation and urgent testing.
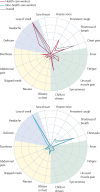
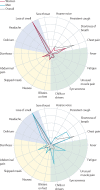
Methods: In this large-scale, prospective, epidemiological surveillance study, we used prospective, observational, longitudinal, self-reported data from participants in the UK on 19 symptoms over 3 days after symptoms onset and COVID-19 PCR test results extracted from the COVID-19 Symptom Study mobile phone app. We divided the study population into a training set (those who reported symptoms between April 29, 2020, and Oct 15, 2020) and a test set (those who reported symptoms between Oct 16, 2020, and Nov 30, 2020), and used three models to analyse the self-reported symptoms: the UK's National Health Service (NHS) algorithm, logistic regression, and the hierarchical Gaussian process model we designed to account for several important variables (eg, specific COVID-19 symptoms, comorbidities, and clinical information). Model performance to predict COVID-19 positivity was compared in terms of sensitivity, specificity, and area under the receiver operating characteristic curve (AUC) in the test set. For the hierarchical Gaussian process model, we also evaluated the relevance of symptoms in the early detection of COVID-19 in population subgroups stratified according to occupation, sex, age, and body-mass index.
Findings: The training set comprised 182 991 participants and the test set comprised 15 049 participants. When trained on 3 days of self-reported symptoms, the hierarchical Gaussian process model had a higher prediction AUC (0·80 [95% CI 0·80-0·81]) than did the logistic regression model (0·74 [0·74-0·75]) and the NHS algorithm (0·67 [0·67-0·67]). AUCs for all models increased with the number of days of self-reported symptoms, but were still high for the hierarchical Gaussian process model at day 1 (0·73 [95% CI 0·73-0·74]) and day 2 (0·79 [0·78-0·79]). At day 3, the hierarchical Gaussian process model also had a significantly higher sensitivity, but a non-statistically lower specificity, than did the two other models. The hierarchical Gaussian process model also identified different sets of relevant features to detect COVID-19 between younger and older subgroups, and between health-care workers and non-health-care workers. When used during different pandemic periods, the model was robust to changes in populations.
Interpretation: Early detection of SARS-CoV-2 infection is feasible with our model. Such early detection is crucial to contain the spread of COVID-19 and efficiently allocate medical resources.
Funding: ZOE, the UK Government Department of Health and Social Care, the Wellcome Trust, the UK Engineering and Physical Sciences Research Council, the UK National Institute for Health Research, the UK Medical Research Council, the British Heart Foundation, the Alzheimer's Society, the Chronic Disease Research Foundation, and the Massachusetts Consortium on Pathogen Readiness.
Copyright © 2021 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests ATC reports personal fees from Pfizer, Bayer Pharma, and Boehringer Ingelheim, outside the submitted work. CJS reports grants from the Chronic Disease Research Foundation, during the conduct of the study. JCP, LP, JW, and TS report other (work) and consultancy from ZOE, during the conduct of the study. CHS reports grants from the Alzheimer's Society, during the conduct of the study. DAD reports grants from the National Institutes of Health during the conduct of the study and has previously served as a co-investigator on an unrelated trial supported by ZOE. RD reports grants from the Department of Health and Social Care, during the conduct of the study, and personal fees from ZOE, outside the submitted work. SO reports grants from the Wellcome Trust, Innovate UK Research and Innovation, and the Chronic Disease Research Foundation, during the conduct of the study. All other authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

