Loss of p16/Ink4a drives high frequency of rhabdomyosarcoma in a rat model of Duchenne muscular dystrophy
- PMID: 34334511
- PMCID: PMC8498826
- DOI: 10.1292/jvms.21-0243
Loss of p16/Ink4a drives high frequency of rhabdomyosarcoma in a rat model of Duchenne muscular dystrophy
Abstract
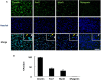
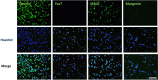
Rhabdomyosarcoma (RMS) is an aggressive type of soft tissue sarcoma, and pleomorphic RMS is a rare subtype of RMS found in adult. p16 is a tumor suppressor which inhibits cell cycle. In human RMS, p16 gene is frequently deleted, but p16-null mice do not develop RMS. We reported that genetic ablation of p16 by the crossbreeding of p16 knock-out rats (p16-KO rats) improved the dystrophic phenotype of a rat model of Duchenne muscular dystrophy (Dmd-KO rats). However, p16/Dmd double knock-out rats (dKO rats) unexpectedly developed sarcoma. In the present study, we raised p16-KO, Dmd-KO, and dKO rats until 11 months of age. Twelve out of 22 dKO rats developed pleomorphic RMS after 9 months of age, while none of p16-KO rats and Dmd-KO rats developed tumor. The neoplasms were connected to skeletal muscle tissue with indistinct borders and characterized by diffuse proliferation of pleomorphic cells which had eosinophilic cytoplasm and atypical nuclei with anisokaryosis. For almost all cases, the tumor cells immunohistochemically expressed myogenic markers including desmin, MyoD, and myogenin. The single cell cloning from tumor primary cells gained 20 individual Pax7-negative MyoD-positive RMS cell clones. Our results demonstrated that double knock-out of p16 and dystrophin in rats leads to the development of pleomorphic RMS, providing an animal model that may be useful to study the developmental mechanism of pleomorphic RMS.
Keywords: MyoD; desmin; muscular dystrophy; rhabdomyosarcoma; skeletal muscle.
Conflict of interest statement
The authors have nothing to disclose.
Figures


Similar articles
-
Muscle Stem Cells Give Rise to Rhabdomyosarcomas in a Severe Mouse Model of Duchenne Muscular Dystrophy.Cell Rep. 2019 Jan 15;26(3):689-701.e6. doi: 10.1016/j.celrep.2018.12.089. Cell Rep. 2019. PMID: 30650360 Free PMC article.
-
Differential metabolic secretion between muscular dystrophy mouse-derived spindle cell sarcomas and rhabdomyosarcomas drives tumor type development.Am J Physiol Cell Physiol. 2024 Jul 1;327(1):C34-C47. doi: 10.1152/ajpcell.00523.2023. Epub 2024 Apr 22. Am J Physiol Cell Physiol. 2024. PMID: 38646787
-
Alterations in Notch signalling in skeletal muscles from mdx and dko dystrophic mice and patients with Duchenne muscular dystrophy.Exp Physiol. 2014 Apr;99(4):675-87. doi: 10.1113/expphysiol.2013.077255. Epub 2014 Jan 17. Exp Physiol. 2014. PMID: 24443351
-
Muscle stem cell dysfunction in rhabdomyosarcoma and muscular dystrophy.Curr Top Dev Biol. 2024;158:83-121. doi: 10.1016/bs.ctdb.2024.01.019. Epub 2024 Feb 19. Curr Top Dev Biol. 2024. PMID: 38670717 Review.
-
The Duchenne muscular dystrophy gene and cancer.Cell Oncol (Dordr). 2021 Feb;44(1):19-32. doi: 10.1007/s13402-020-00572-y. Epub 2020 Nov 14. Cell Oncol (Dordr). 2021. PMID: 33188621 Free PMC article. Review.
References
-
- Barr F. G., Nascimentro A. F., Montgomery E. A., Parham D. M.2013. Skeletal-muscle tumours. pp. 123–135. In: WHO clasification of tumours of soft tissue and bone, 4th ed. (Fletcher, C. D. M., Bridge, J. A., Hogendoorn, P. C. W. and Mertens, F. eds.), IARC Press, Lyon.
-
- Blum J. M., Añó L., Li Z., Van Mater D., Bennett B. D., Sachdeva M., Lagutina I., Zhang M., Mito J. K., Dodd L. G., Cardona D. M., Dodd R. D., Williams N., Ma Y., Lepper C., Linardic C. M., Mukherjee S., Grosveld G. C., Fan C. M., Kirsch D. G.2013. Distinct and overlapping sarcoma subtypes initiated from muscle stem and progenitor cells. Cell Rep. 5: 933–940. doi: 10.1016/j.celrep.2013.10.020 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

