Effect on Plasma Protein S Activity in Patients Receiving the Factor Xa Inhibitors
- PMID: 34334529
- PMCID: PMC9252639
- DOI: 10.5551/jat.62951
Effect on Plasma Protein S Activity in Patients Receiving the Factor Xa Inhibitors
Abstract
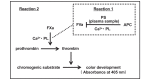
Aims: Measurement of protein S (PS) activity in patients taking direct oral anticoagulants (DOACs) using reagents based on a clotting assay results in falsely high PS activity, thus masking inherited PS deficiency, which is most frequently seen in the Japanese population. In this study, we investigated the effect of factor Xa (FXa) inhibitors on PS activity using the reagent on the basis of the chromogenic assay, which was recently developed in Japan.
Methods: The study enrolled 152 patients (82 males and 70 females; the average age: 68.5±14.0 years) receiving three FXa inhibitors (rivaroxaban, edoxaban, and apixaban). PS activity was measured using the reagents on the basis of the clotting and chromogenic assays.
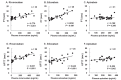
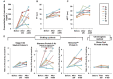
Results: PS activity measured by the clotting assay reagents exhibited falsely high values depending on the plasma concentrations of FXa inhibitors in patients taking either rivaroxaban or edoxaban. However, none of the three FXa inhibitors affected PS activity when measured using the chromogenic assay.
Conclusion: In patients taking rivaroxaban or edoxaban, inherited PS deficiency is likely missed because the levels of PS activity measured using the reagents based on the clotting assay are falsely high. However, we report that three FXa inhibitors do not affect PS activity measured by the chromogenic assay. When measuring the levels of PS activity in patients undergoing DOACs, the principles of each reagent should be understood. Furthermore, plasma samples must be collected at the time when plasma concentrations of DOACs are lowest or the DOAC-Stop reagent should be used.
Keywords: Chromogenic assay; Clotting assay; Factor Xa inhibitor; Inherited protein S deficiency; Protein S Tokushima.
Figures


References
-
- Castellucci LA, Cameron C, Le Gal G, Rodger MA., Coyle D, Wells PS, Clifford T, Gandara E, Wells G, Carrier M: Clinical and safety outcomes associated with treatment of acute venous thromboembolism: a systematic review and meta-analysis. JAMA, 2014; 312: 1122-1135 - PubMed
-
- Verhamme P, Wells PS, Segers A, Ageno W, Brekelmans MP, Cohen AT, Meyer G, Grosso MA, Raskob G, Weitz JI, Zhang G, Buller H: Dose reduction of edoxaban preserves efficacy and safety for the treatment of venous thromboembolism. An analysis of the randomised, double-blind HOKUSAI VTE trial. Thromb Haemost, 2016; 116: 747-753 - PubMed
-
- Almutairi AR, Zhou L, Gellad WF, Lee JK, Slack MK, Martin JR, Lo-Ciganic W-H: Effectiveness and Safety of Non-vitamin K Antagonist Oral Anticoagulants for Atrial Fibrillation and Venous Thromboembolism: A Systematic Review and Meta-analyses. Clin Ther, 2017; 39: 1456-1478 - PubMed
-
- Nick van Es, Coppens M, Schulman S, Middeldorp S, Büller HR: Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood, 2014; 124: 1968-1975 - PubMed

