Liquid-liquid phase separation in human health and diseases
- PMID: 34334791
- PMCID: PMC8326283
- DOI: 10.1038/s41392-021-00678-1
Liquid-liquid phase separation in human health and diseases
Abstract
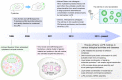
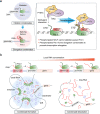
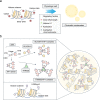
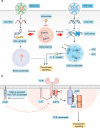
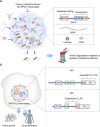
Emerging evidence suggests that liquid-liquid phase separation (LLPS) represents a vital and ubiquitous phenomenon underlying the formation of membraneless organelles in eukaryotic cells (also known as biomolecular condensates or droplets). Recent studies have revealed evidences that indicate that LLPS plays a vital role in human health and diseases. In this review, we describe our current understanding of LLPS and summarize its physiological functions. We further describe the role of LLPS in the development of human diseases. Additionally, we review the recently developed methods for studying LLPS. Although LLPS research is in its infancy-but is fast-growing-it is clear that LLPS plays an essential role in the development of pathophysiological conditions. This highlights the need for an overview of the recent advances in the field to translate our current knowledge regarding LLPS into therapeutic discoveries.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

