Recent Advances in the Selective Oxidative Dearomatization of Phenols to o-Quinones and o-Quinols with Hypervalent Iodine Reagents
- PMID: 34334960
- PMCID: PMC8323659
- DOI: 10.1055/s-0037-1610760
Recent Advances in the Selective Oxidative Dearomatization of Phenols to o-Quinones and o-Quinols with Hypervalent Iodine Reagents
Abstract
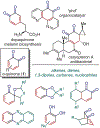
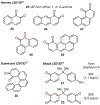
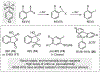
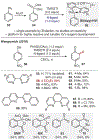
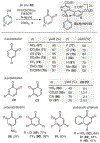
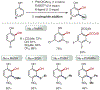
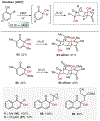
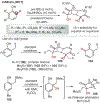
ortho-Quinones are valuable molecular frameworks with diverse applications across biology, materials, organic synthesis, catalysis, and coordination chemistry. Despite their broad utility, their synthesis remains challenging, in particular via the direct oxidation of readily accessible phenols, due to the need to affect regioselective ortho oxidation coupled with the sensitivity of the resulting o-quinone products. The perspective looks at the emergence of I(V) hypervalent iodine reagents as an effective class of oxidants for regioselective o-quinone synthesis. The application of these reagents in regioselective phenol oxidation to both o-quinones and o-quinols will be discussed, including a recent report from our laboratory on the first method for the oxidation of electron-deficient phenols using a novel nitrogen-ligated I(V) reagent. Also included are select examples of total syntheses utilizing this methodology as well as recent advancements in chiral I(V) reagent design for asymmetric phenol dearomatization.
Keywords: I(V) reagent; hypervalent iodine; ortho-quinol; ortho-quinone; phenol dearomatization.
Figures


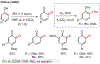

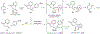
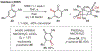

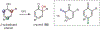
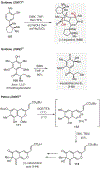
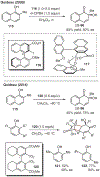
References
-
- Land EJ; Ramsden CA; Riley PA In Melanins and Mela-nosomes: Biosynthesis, Structure, Physiological and Pathological Functions; Borovansky J; Riley PA, Ed.; Wiley-VCH: Weinheim, 2011, 63–86.
- Bruins JJ; Albada B; van Delft F Chem. Eur. J 2018, 24, 4749. - PMC - PubMed
- Saruul E; Murata T; Selenge E; Sasaki K; Yoshizaki F; Batkhuu J Bioorg. Med. Chem. Lett 2015, 25, 2555. - PubMed
- Bian J; Li X; Wang N; Wu X; You Q; Zhang X Eur. J. Med. Chem 2017, 129, 27. - PubMed
- McSkimming A; Cheisson T; Carrol PJ; Schelter EJ J. Am. Chem. Soc 2018, 140, 1223. - PubMed
-
- Liao C-C; Peddinti RK Acc. Chem. Res 2002, 35, 856. - PubMed
- Magdziak D; Meek SJ; Pettus TR R. Chem. Rev 2004, 104, 1383. - PubMed
- Ramsden CA Adv. Heterocycl. Chem 2010, 100, 1.
- Nair V; Menon RS; Biju AT; Abhilash KG Chem. Soc. Rev 2012, 41, 1050. - PubMed
- Radhika S; Saranya S; Harry NA; Anilkuman G ChemistrySelect 2019, 4, 9124.
- Harry NA; Saranya S; Krishnan KK; Anilkumar G Asian J. Org. Chem 2017, 6, 945.
-
- Kharisov BI; Mendez-Rojas MA; Garnovskii AD; Ivakhnenko EP; Ortiz-Mendez U J. Coord. Chem 2002, 55, 745.
Grants and funding
LinkOut - more resources
Full Text Sources
