Influence of SULT1A1*2 Polymorphism on Plasma Efavirenz Concentration in Thai HIV-1 Patients
- PMID: 34335044
- PMCID: PMC8318725
- DOI: 10.2147/PGPM.S306358
Influence of SULT1A1*2 Polymorphism on Plasma Efavirenz Concentration in Thai HIV-1 Patients
Abstract
Purpose: Plasma efavirenz (EFV) concentrations within therapeutic levels are essential to successfully treat patients suffering from human immunodeficiency virus (HIV) type 1. In addition to the drug-metabolizing enzyme CYP2B6, other phase II drug-metabolizing enzymes and transporters may have an important role in the pharmacokinetics of EFV. Thus, the influence of phase II drug-metabolizing enzymes and drug transporters on plasma EFV levels was investigated in Thai HIV patients receiving EFV.
Patients and methods: Genotyping was performed by TaqMan® real-time PCR in 149 HIV-infected Thai adults, and plasma efavirenz concentration was measured by a validated high-performance liquid chromatography in 12 hours after dosing steady-state plasma samples at week 12 and 24.
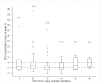
Results: Patients with three or more copies of SULT1A1 had significantly lower median plasma EFV concentrations than those carrying two copies at week 12 (p=0.046) and SULT1A1*2 (c.638G>A) carriers had significantly lower median plasma EFV concentrations compared to those not carrying the variant at week 24 (p=0.048). However, no significant association was found after adjusting for CYP2B6 genotype.
Conclusion: Genetic variation in a combination of SULT1A1*2 and SULT1A1 copy number may contribute to variability in EFV metabolism and thereby may impact drug response. The influence of a combination between the SULT1A1 and CYP2B6 genotype on EFV pharmacokinetics should be further investigated in a larger study population.
Keywords: HIV-1; Thai; efavirenz; phase II drug-metabolizing enzymes; transporter genes.
© 2021 Chamnanphon et al.
Conflict of interest statement
The authors reported no conflicts of interest in this work.
Figures

References
-
- Barusrux S, Urwijitaroon Y, Puapairoj C, Romphruk A, Sriwanitchrak P. Association of HCV and Treponema pallidum infection in HIV infected northeastern Thai male blood donors. J Med Assoc Thai. 1997;80(Suppl 1):S106–111. - PubMed
-
- Langmann P, Weissbrich B, Desch S, et al. Efavirenz plasma levels for the prediction of treatment failure in heavily pretreated HIV-1 infected patients. Eur J Med Res. 2002;7(7):309–314. - PubMed
LinkOut - more resources
Full Text Sources

