Common Clinical Practice for Opioid-Induced Constipation: A Physician Survey
- PMID: 34335054
- PMCID: PMC8318709
- DOI: 10.2147/JPR.S318564
Common Clinical Practice for Opioid-Induced Constipation: A Physician Survey
Abstract
Background: Opioid-induced constipation (OIC) remains an important clinical obstacle despite the availability of several guidelines and pharmacological options for its management. Here, we surveyed common practices and perceptions about OIC among physicians who prescribe opioids in Italy.
Methods: The online survey included 26 questions about OIC. Responses were analyzed descriptively and aggregated by physician specialty.
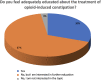
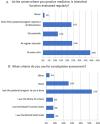
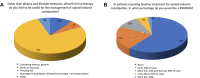
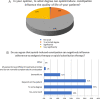
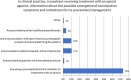
Results: A total of 501 physicians completed the survey. Most respondents (67%) did not feel adequately educated about OIC despite general consensus regarding interest in the topic. Overall, 62-75% of physicians regularly evaluated intestinal function or OIC symptoms in patients receiving opioid therapy. The most common method for assessment was patient diary; few physicians used a validated instrument such as the Rome IV criteria. Psychiatrists and addiction specialists showed the lowest interest and poorest practices. Most respondents (78%) preferred macrogol prophylaxis followed by macrogol plus another laxative for first-line treatment of OIC symptoms. Peripheral-acting mu opioid receptor antagonists (PAMORAs) were not widely used among physicians; 61% had never prescribed a PAMORA for OIC.
Conclusion: Our findings reveal important differences in clinical practice for OIC across physician specialties. Additional formative efforts are necessary to improve awareness about best practices in OIC.
Keywords: chronic pain; opioid; opioid-induced constipation; peripherally acting mu opioid receptor antagonist.
© 2021 Coluzzi et al.
Conflict of interest statement
FC is a speaker and consultant for Angelini, Grunenthal, Malesci, Molteni, Pfizer, and Shionogi. DA has received research grants and consultant fees from Intercept Pharma, Molteni, Shionogi, and Vesta, and is a consultant for Aboca. ATC has received research grants from Molteni, Gruenenthal GmbH, Prostrakan, Amgen, and Ipsen and is a consultant for Kyowa Kirin, Gruenenthal GmbH, Pfizer, Helsinn Healthcare, Molteni, Shionogi, Italfarmaco, Sandoz International GmbH, Angelini Holding S.p.A., Mundipharma, and the Institute de Recherche “Pierre Fabre.” WG has received research grants and other funding from Molteni and Shionogi. FM has received research grants and other funding from Molteni and Shionogi. GM has received research grants and other funding from Molteni and Shionogi. CP has received research grants and other funding from Molteni and Shionogi. GV is Member of the Advisor Boards of Abbott, Dompé, Malesci, Menarini International, Molteni, Mundipharma, Shionogi. Also, he is Member of the Speakers’ Bureau of Berlin-Chemie, Dompé, MAP, Menarini International, MCAC, Molteni, Takeda. He has received funds for research by Dompé, Fondazione Maugeri and Pfizer. He is a Member of the Editorial Board of several scientific journals, and is Editor in Chief of Pain and Therapy. FL has received research grants and other funding from Molteni and Shionogi. The authors report no other conflicts of interest in this work.
Figures

References
-
- Glare P, Walsh D, Sheehan D. The adverse effects of morphine: a prospective survey of common symptoms during repeated dosing for chronic cancer pain. Am J Hosp Palliat Care. 2006. 23(3):229–235. - PubMed
-
- Drewes AM, Munkholm P, Simrén M, et al. Definition, diagnosis and treatment strategies for opioid-induced bowel dysfunction-Recommendations of the Nordic Working Group. Scand J Pain. 2016;11:111–122. - PubMed
-
- Tuteja AK, Biskupiak J, Stoddard GJ, Lipman AG. Opioid-induced bowel disorders and narcotic bowel syndrome in patients with chronic non-cancer pain. Neurogastroenterol Motil. 2010;22(4):e96. - PubMed
-
- Bell T, Annunziata K, Leslie JB. Opioid-induced constipation negatively impacts pain management, productivity, and health-related quality of life: findings from the National Health and Wellness Survey. J Opioid Manag. 5(3):137–144. - PubMed

