Effects of Wearable Transcutaneous Electrical Nerve Stimulation on Fibromyalgia: A Randomized Controlled Trial
- PMID: 34335055
- PMCID: PMC8318714
- DOI: 10.2147/JPR.S316371
Effects of Wearable Transcutaneous Electrical Nerve Stimulation on Fibromyalgia: A Randomized Controlled Trial
Abstract
Purpose: Fibromyalgia is a chronic condition characterized by widespread pain and interference with daily activities. The aim of this study is to assess the benefit of transcutaneous electrical nerve stimulation (TENS) for persons diagnosed with fibromyalgia.
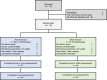
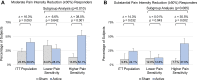
Patients and methods: Adults meeting diagnostic criteria for fibromyalgia were randomized in a double-blind trial to receive either an active (n=62) or sham (n=57) wearable TENS device for 3-months. Subjects were classified as having lower or higher pain sensitivity by Quantitative Sensory Testing (QST). Patient Global Impression of Change (PGIC, primary outcome) and secondary efficacy measures including Fibromyalgia Impact Questionnaire (FIQR), Brief Pain Inventory (BPI) and painDETECT questionnaire (PDQ) were assessed at baseline, 6-weeks and 3-months. Treatment effects were determined by a mixed model for repeated measures (MMRM) analysis of the intention-to-treat (ITT) population (N=119). A pre-specified subgroup analysis of pain sensitivity was conducted using an interaction term in the model.
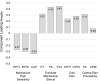
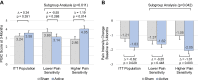
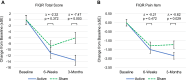
Results: No differences were found between active and sham treatment on PGIC scores at 3-months (0.34, 95% CI [-0.37, 1.04], p=0.351) in the ITT population. However, in subjects with higher pain sensitivity (n=60), PGIC was significantly greater for active treatment compared to sham (1.19, 95% CI [0.24, 2.13], p=0.014). FIQR total score (-7.47, 95% CI [-12.46, -2.48], p=0.003), FIQR pain item (-0.62, 95% CI [-1.17, -0.06], p=0.029), BPI Interference (-0.70, 95% CI [-1.30, -0.11], p=0.021) and PDQ (-1.69, 95% CI [-3.20, -0.18], p=0.028) exhibited significant improvements for active treatment compared to sham in the ITT population. Analgesics use was stable and comparable in both groups.
Conclusion: This study demonstrated modest treatment effects of reduced disease impact, pain and functional impairment from wearable TENS in individuals with fibromyalgia. Subjects with higher pain sensitivity exhibited larger treatment effects than those with lower pain sensitivity. Wearable TENS may be a safe treatment option for people with fibromyalgia.
Clinicaltrialsgov registration: NCT03714425.
Keywords: clinical trial; fibromyalgia; neuromodulation; non-pharmacological treatment; transcutaneous electrical nerve stimulation; wearable.
© 2021 Jamison et al.
Conflict of interest statement
S. Gozani is an employee and shareholder of NeuroMetrix, Inc. He holds multiple patents related to the Quell device. CJ Gilligan reports Sponsored Research from Mainstay Medical and Sollis, personal fees from Medtronic, personal fees from Abbott, personal fees from Saluda, personal fees from Persica, outside the submitted work. The remaining authors report no potential conflicts of interest for this work.
Figures
Similar articles
-
Higher Pain Sensitivity Predicts Efficacy of a Wearable Transcutaneous Electrical Nerve Stimulation Device for Persons With Fibromyalgia: A Randomized Double-Blind Sham-Controlled Trial.Neuromodulation. 2022 Dec;25(8):1410-1420. doi: 10.1111/ner.13463. Epub 2022 Jun 14. Neuromodulation. 2022. PMID: 34056781 Clinical Trial.
-
Transcutaneous electrical nerve stimulation for fibromyalgia-like syndrome in patients with Long-COVID: a pilot randomized clinical trial.Sci Rep. 2024 Nov 8;14(1):27224. doi: 10.1038/s41598-024-78651-5. Sci Rep. 2024. PMID: 39516528 Free PMC article. Clinical Trial.
-
The effect of the EXOPULSE Mollii Suit on pain and fibromyalgia-related symptoms-A randomized sham-controlled crossover trial.Eur J Pain. 2025 Feb;29(2):e4729. doi: 10.1002/ejp.4729. Epub 2024 Sep 18. Eur J Pain. 2025. PMID: 39291602 Free PMC article. Clinical Trial.
-
Efficacy and safety of duloxetine versus placebo in adolescents with juvenile fibromyalgia: results from a randomized controlled trial.Pediatr Rheumatol Online J. 2019 May 28;17(1):27. doi: 10.1186/s12969-019-0325-6. Pediatr Rheumatol Online J. 2019. PMID: 31138224 Free PMC article. Clinical Trial.
-
[Analgesic effects of transcutaneous electrical nerve stimulation (TENS) in patients with fibromyalgia: A systematic review].Aten Primaria. 2019 Aug-Sep;51(7):406-415. doi: 10.1016/j.aprim.2018.03.010. Epub 2018 Jul 17. Aten Primaria. 2019. PMID: 30029964 Free PMC article. Spanish.
Cited by
-
Quantitative Sensory Testing in Fibromyalgia Syndrome: A Scoping Review.Biomedicines. 2025 Apr 17;13(4):988. doi: 10.3390/biomedicines13040988. Biomedicines. 2025. PMID: 40299678 Free PMC article. Review.
-
Influence of Transcutaneous Electrical Nerve Stimulation (TENS) on Pressure Pain Thresholds and Conditioned Pain Modulation in a Randomized Controlled Trial in Women With Fibromyalgia.J Pain. 2024 Jun;25(6):104452. doi: 10.1016/j.jpain.2023.12.009. Epub 2023 Dec 26. J Pain. 2024. PMID: 38154621 Free PMC article. Clinical Trial.
-
Comparative efficacy of neuromodulation and structured exercise program on pain and muscle oxygenation in fibromyalgia patients: a randomized crossover study.Front Physiol. 2024 Jul 23;15:1414100. doi: 10.3389/fphys.2024.1414100. eCollection 2024. Front Physiol. 2024. PMID: 39108537 Free PMC article.
-
Physical Modalities for the Treatment of Pain in Patients with Fibromyalgia.Mediterr J Rheumatol. 2025 Mar 31;36(1):12-27. doi: 10.31138/mjr.041124.pht. eCollection 2025 Mar. Mediterr J Rheumatol. 2025. PMID: 40557176 Free PMC article. Review.
-
Comparative Efficacy of Neuromodulation and Structured Exercise Program on Autonomic Modulation in Fibromyalgia Patients: Pilot Study.J Clin Med. 2024 Jul 23;13(15):4288. doi: 10.3390/jcm13154288. J Clin Med. 2024. PMID: 39124555 Free PMC article.
References
Associated data
LinkOut - more resources
Full Text Sources
Medical

