Overexpression of TOLLIP Protects against Acute Kidney Injury after Paraquat Intoxication through Inhibiting NLRP3 Inflammasome Activation Modulated by Toll-Like Receptor 2/4 Signaling
- PMID: 34335089
- PMCID: PMC8298172
- DOI: 10.1155/2021/5571272
Overexpression of TOLLIP Protects against Acute Kidney Injury after Paraquat Intoxication through Inhibiting NLRP3 Inflammasome Activation Modulated by Toll-Like Receptor 2/4 Signaling
Abstract
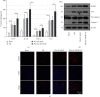
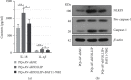
Paraquat (PQ) can cause multiorgan failure including acute kidney injury (AKI). Our prior study showed that Toll-interacting protein (TOLLIP) protected against PQ-induced acute lung injury. However, the role of TOLLIP in PQ-induced AKI remains undefined. This study was aimed at understanding the role and mechanism of TOLLIP in AKI. Six-eight-week-old male Wistar rats were intraperitoneally injected with 25 mg/kg PQ to induce AKI for 24 h in vivo. HK-2 cells were treated with 300 μM PQ for 24 h to induce cellular injury in vitro or 300 μM PQ and 5 μM nuclear factor-κB (NF-κB) inhibitor BAY11-7082 for 24 h. Rats were infected with adenovirus carrying TOLLIP shRNA via tail vein injection and HK-2 cells with adenovirus carrying TOLLIP shRNA or TOLLIP 48 h before PQ exposure. Results showed that TOLLIP and Toll-like receptor 2/4 (TLR2/4) expressions were boosted in the kidney after PQ intoxication. The toxic effect of PQ on the kidney and HK-2 cells was exacerbated by TOLLIP knockdown, as evidenced by aggravated glomerulus and tubule injury, inflammatory infiltration, and cell apoptosis in the kidney and increased loss of cell viability and apoptotic cells in HK-2 cells. TOLLIP knockdown also enhanced PQ-induced NLR family pyrin domain-containing 3 (NLRP3) inflammasome activation in vivo and in vitro and TLR2/4-NF-κB signaling in vitro, reflected by increased contents of proinflammatory cytokines and expressions of NLRP3 inflammasome-related proteins in the kidney and HK-2 cells and expressions of TLR2, TLR4, and nuclear NF-κB p65 in HK-2 cells. However, TOLLIP overexpression inhibited PQ-induced loss of cell viability, cell apoptosis, NLRP3 inflammasome activation, and TLR2/4-NF-κB signaling in vitro. Additionally, BAY11-7082 abolished TOLLIP knockdown-induced NLRP3 inflammasome activation in vitro, indicating that TOLLIP protected against NLRP3 inflammasome activation in PQ-induced AKI through inhibiting TLR2/4-NF-κB signaling. This study highlights the importance of TOLLIP in AKI after PQ intoxication.
Copyright © 2021 Qiang Zheng et al.
Conflict of interest statement
The authors declare there is no conflict of interest regarding the publication of this paper.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

