Relacorilant, a Selective Glucocorticoid Receptor Modulator, Induces Clinical Improvements in Patients With Cushing Syndrome: Results From A Prospective, Open-Label Phase 2 Study
- PMID: 34335465
- PMCID: PMC8317576
- DOI: 10.3389/fendo.2021.662865
Relacorilant, a Selective Glucocorticoid Receptor Modulator, Induces Clinical Improvements in Patients With Cushing Syndrome: Results From A Prospective, Open-Label Phase 2 Study
Erratum in
-
Corrigendum: Relacorilant, a Selective Glucocorticoid Receptor Modulator, Induces Clinical Improvements in Patients With Cushing Syndrome: Results From A Prospective, Open-Label Phase 2 Study.Front Endocrinol (Lausanne). 2022 Apr 27;13:899616. doi: 10.3389/fendo.2022.899616. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35574017 Free PMC article.
Abstract
Introduction/purpose: Relacorilant is a selective glucocorticoid receptor modulator (SGRM) with no progesterone receptor activity. We evaluated the efficacy and safety of relacorilant in patients with endogenous Cushing syndrome (CS).
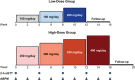
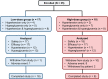
Materials and methods: A single-arm, open-label, phase 2, dose-finding study with 2 dose groups (NCT02804750, https://clinicaltrials.gov/ct2/show/NCT02804750) was conducted at 19 sites in the U.S. and Europe. Low-dose relacorilant (100-200 mg/d; n = 17) was administered for 12 weeks or high-dose relacorilant (250-400 mg/d; n = 18) for 16 weeks; doses were up-titrated by 50 mg every 4 weeks. Outcome measures included proportion of patients with clinically meaningful changes in hypertension and/or hyperglycemia from baseline to last observed visit. For patients with hypertension, clinical response was defined as a ≥5-mmHg decrease in mean systolic or diastolic blood pressure, measured by a standardized and validated 24-h ABPM. For patients with hyperglycemia, clinical response was defined ad-hoc as ≥0.5% decrease in HbA1c, normalization or ≥50-mg/dL decrease in 2-h plasma glucose value on oral glucose tolerance test, or decrease in daily insulin (≥25%) or sulfonylurea dose (≥50%).
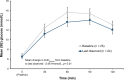
Results: 35 adults with CS and hypertension and/or hyperglycemia (impaired glucose tolerance or type 2 diabetes mellitus) were enrolled, of which 34 (24 women/10 men) received treatment and had postbaseline data. In the low-dose group, 5/12 patients (41.7%) with hypertension and 2/13 patients (15.4%) with hyperglycemia achieved response. In the high-dose group, 7/11 patients (63.6%) with hypertension and 6/12 patients (50%) with hyperglycemia achieved response. Common (≥20%) adverse events included back pain, headache, peripheral edema, nausea, pain at extremities, diarrhea, and dizziness. No drug-induced vaginal bleeding or hypokalemia occurred.
Conclusions: The SGRM relacorilant provided clinical benefit to patients with CS without undesirable antiprogesterone effects or drug-induced hypokalemia.
Keywords: Cushing syndrome; clinical trial; cortisol; glucocorticoid; hypercortisolism; hyperglycemia; hypertension; relacorilant.
Copyright © 2021 Pivonello, Bancos, Feelders, Kargi, Kerr, Gordon, Mariash, Terzolo, Ellison and Moraitis.
Conflict of interest statement
The authors declare that this study received funding from Corcept Therapeutics (Menlo Park, CA, USA). The funder had a role in study design, data collection and analysis, and AM, as an author of the manuscript and employee of Corcept Therapeutics, had a role in the study design, the decision to publish, the interpretation of clinical data, the revision of the manuscript, and approval of the final manuscript to submit. Open Access publication fees were paid by Corcept Therapeutics. RP: Consultant: Ferring, Ipsen, Novartis, Pfizer, ViroPharma-Shire; Speaker: Novartis, ViroPharma-Shire; Research support: Corcept Therapeutics, Novartis, ViroPharma-Shire; Grant support: IBSA, Novartis, Pfizer, ViroPharma-Shire. IB: Consultant: HRA Pharma, Sparrow Pharmaceutics, Strongbridge; Data and Safety Monitoring Panel, Adrenas. RF: Consultant: Corcept Therapeutics; Speaker: HRA Pharma. AK: Consultant: Strongbridge; Research support: Corcept Therapeutics. MG: Research support: Corcept Therapeutics, Crinetics, Ionis, Ipsen, Novartis, Novo Nordisk, Opko, Strongbridge, Teva. CM: Consultant: Horizon Therapeutics; Research support: Corcept Therapeutics, Eli Lilly, Medtronic. MT: Consultant: HRA Pharma; Research support: Corcept Therapeutics. NE: Consultant: Corcept Therapeutics, Pentara, Trialwise. AM: Employee: Corcept Therapeutics. The remaining author (JK) declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer AALC declared a shared affiliation with one of the authors, RP, to the handling editor at time of review. Author NE was employed by company Trialwise.
Figures

Similar articles
-
Management and Medical Therapy of Mild Hypercortisolism.Int J Mol Sci. 2021 Oct 26;22(21):11521. doi: 10.3390/ijms222111521. Int J Mol Sci. 2021. PMID: 34768949 Free PMC article. Review.
-
Glucocorticoid Receptor Antagonism Upregulates Somatostatin Receptor Subtype 2 Expression in ACTH-Producing Neuroendocrine Tumors: New Insight Based on the Selective Glucocorticoid Receptor Modulator Relacorilant.Front Endocrinol (Lausanne). 2022 Jan 4;12:793262. doi: 10.3389/fendo.2021.793262. eCollection 2021. Front Endocrinol (Lausanne). 2022. PMID: 35058882 Free PMC article.
-
Relacorilant, a Selective Glucocorticoid Receptor Modulator in Development for the Treatment of Patients With Cushing Syndrome, Does Not Cause Prolongation of the Cardiac QT Interval.Endocr Pract. 2024 Jan;30(1):11-18. doi: 10.1016/j.eprac.2023.09.011. Epub 2023 Oct 5. Endocr Pract. 2024. PMID: 37805100
-
Mifepristone, a glucocorticoid receptor antagonist, produces clinical and metabolic benefits in patients with Cushing's syndrome.J Clin Endocrinol Metab. 2012 Jun;97(6):2039-49. doi: 10.1210/jc.2011-3350. Epub 2012 Mar 30. J Clin Endocrinol Metab. 2012. PMID: 22466348 Clinical Trial.
-
Advances in the medical treatment of Cushing's syndrome.Lancet Diabetes Endocrinol. 2019 Apr;7(4):300-312. doi: 10.1016/S2213-8587(18)30155-4. Epub 2018 Jul 20. Lancet Diabetes Endocrinol. 2019. PMID: 30033041 Review.
Cited by
-
Management and Medical Therapy of Mild Hypercortisolism.Int J Mol Sci. 2021 Oct 26;22(21):11521. doi: 10.3390/ijms222111521. Int J Mol Sci. 2021. PMID: 34768949 Free PMC article. Review.
-
Individualized medical treatment options in Cushing disease.Front Endocrinol (Lausanne). 2022 Dec 2;13:1060884. doi: 10.3389/fendo.2022.1060884. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36531477 Free PMC article. Review.
-
Relacorilant or surgery improved hemostatic markers in Cushing syndrome.J Endocrinol Invest. 2025 Mar;48(3):671-680. doi: 10.1007/s40618-024-02468-2. Epub 2024 Sep 21. J Endocrinol Invest. 2025. PMID: 39305441 Free PMC article. Clinical Trial.
-
Peripheral glucocorticoid receptor antagonism by relacorilant with modest HPA axis disinhibition.J Endocrinol. 2022 Dec 22;256(2):e220263. doi: 10.1530/JOE-22-0263. Print 2023 Feb 1. J Endocrinol. 2022. PMID: 36445262 Free PMC article.
-
Epidemiology and Management of Hypertension and Diabetes Mellitus in Patients with Mild Autonomous Cortisol Secretion: A Review.Biomedicines. 2023 Nov 22;11(12):3115. doi: 10.3390/biomedicines11123115. Biomedicines. 2023. PMID: 38137336 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

