"Jumping Jack": Genomic Microsatellites Underscore the Distinctiveness of Closely Related Pseudoperonospora cubensis and Pseudoperonospora humuli and Provide New Insights Into Their Evolutionary Past
- PMID: 34335513
- PMCID: PMC8317435
- DOI: 10.3389/fmicb.2021.686759
"Jumping Jack": Genomic Microsatellites Underscore the Distinctiveness of Closely Related Pseudoperonospora cubensis and Pseudoperonospora humuli and Provide New Insights Into Their Evolutionary Past
Erratum in
-
Erratum: "Jumping Jack": Genomic Microsatellites Underscore the Distinctiveness of Closely Related Pseudoperonospora cubensis and Pseudoperonospora humuli and Provide New Insights Into Their Evolutionary Past.Front Microbiol. 2021 Sep 29;12:772633. doi: 10.3389/fmicb.2021.772633. eCollection 2021. Front Microbiol. 2021. PMID: 34659194 Free PMC article.
Abstract
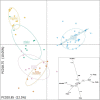
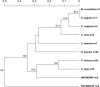
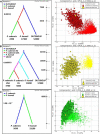
Downy mildews caused by obligate biotrophic oomycetes result in severe crop losses worldwide. Among these pathogens, Pseudoperonospora cubensis and P. humuli, two closely related oomycetes, adversely affect cucurbits and hop, respectively. Discordant hypotheses concerning their taxonomic relationships have been proposed based on host-pathogen interactions and specificity evidence and gene sequences of a few individuals, but population genetics evidence supporting these scenarios is missing. Furthermore, nuclear and mitochondrial regions of both pathogens have been analyzed using microsatellites and phylogenetically informative molecular markers, but extensive comparative population genetics research has not been done. Here, we genotyped 138 current and historical herbarium specimens of those two taxa using microsatellites (SSRs). Our goals were to assess genetic diversity and spatial distribution, to infer the evolutionary history of P. cubensis and P. humuli, and to visualize genome-scale organizational relationship between both pathogens. High genetic diversity, modest gene flow, and presence of population structure, particularly in P. cubensis, were observed. When tested for cross-amplification, 20 out of 27 P. cubensis-derived gSSRs cross-amplified DNA of P. humuli individuals, but few amplified DNA of downy mildew pathogens from related genera. Collectively, our analyses provided a definite argument for the hypothesis that both pathogens are distinct species, and suggested further speciation in the P. cubensis complex.
Keywords: downy mildew; evolution; genotyping; host specificity; obligate pathogens; oomycete; speciation.
Copyright © 2021 Nowicki, Hadziabdic, Trigiano, Boggess, Kanetis, Wadl, Ojiambo, Cubeta, Spring, Thines, Runge and Scheffler.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Agapow P. M., Burt A. (2001). Indices of multilocus linkage disequilibrium. Mol. Ecol. Notes 1 101–102. 10.1046/j.1471-8278.2000.00014.x - DOI
-
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. (1990). Basic local alignment search tool. J. Mol. Biol. 215 403–410. - PubMed
-
- Amos W., Hoffman J., Frodsham A., Zhang L., Best S., Hill A. (2007). Automated binning of microsatellite alleles: problems and solutions. Mol. Ecol. Notes 7 10–14. 10.1111/j.1471-8286.2006.01560.x - DOI
-
- Burkhardt A., Day B. (2013). A genomics perspective on cucurbit-oomycete interactions. Plant Biotechnol. 30 265–271. 10.5511/plantbiotechnology.13.0315a - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

