Safety and efficacy of a feed additive consisting of a flavonoid-rich dried extract of Citrus × aurantium L. fruit (bitter orange extract) for use in all animal species (FEFANA asbl)
- PMID: 34335921
- PMCID: PMC8314171
- DOI: 10.2903/j.efsa.2021.6709
Safety and efficacy of a feed additive consisting of a flavonoid-rich dried extract of Citrus × aurantium L. fruit (bitter orange extract) for use in all animal species (FEFANA asbl)
Abstract
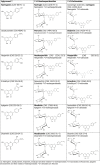
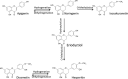
Following a request from the European Commission, the EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) was asked to deliver a scientific opinion on the safety and efficacy of a dried flavonoid-rich extract of Citrus × aurantium L. fruit (bitter orange extract), when used as a sensory additive for all animal species. The use of the additive in feed was not expected to increase the exposure to furocoumarins of those target species that are already fed citrus by-products to a relevant extent (< 5%). For dog, cat and ornamental fish, not normally exposed to citrus by-products, no conclusion could be drawn. The FEEDAP Panel concluded that the additive under assessment is safe up to the maximum proposed use level of 400 mg/kg for veal calf (milk replacer), sheep, goat, horse and salmon. For the other species, the calculated maximum safe concentration in complete feed is 102 mg/kg for chicken for fattening, 151 mg/kg for laying hen, 136 mg/kg for turkey for fattening, 182 mg/kg for piglet, 217 mg/kg for pig for fattening, 268 mg/kg for sow, 259 mg/kg for dairy cow and 161 mg/kg for rabbit. The FEEDAP Panel considered that the use in water for drinking is safe provided that the total daily intake of the additive does not exceed the daily amount that is considered safe when consumed via feed, except dog, cat and ornamental fish. No concerns for consumer safety were identified following the use of the additive up to highest safe level in feed for the target animals. The extract under assessment should be considered as irritant to skin, eyes and the respiratory tract, and as a skin sensitiser. Since the additive contains 5-methoxypsoralen, it may cause phototoxicity. The use of the extract in animal feed under the proposed conditions was not expected to pose a risk for the environment. Bitter orange extract was recognised to flavour food. Since its function in feed would be essentially the same as that in food, no further demonstration of efficacy was considered necessary.
Keywords: (‐)-synephrine; 5‐methoxypsoralen; Citrus × aurantium L.; bitter orange extract; flavonoids; flavouring compounds; safety; sensory additives.
© 2021 European Food Safety Authority. EFSA Journal published by John Wiley and Sons Ltd on behalf of European Food Safety Authority.
Figures
Similar articles
-
Safety and efficacy of a feed additive consisting of an essential oil from the leaves of Citrus × aurantium L. (petitgrain bigarade oil) for use in all animal species (FEFANA asbl).EFSA J. 2021 Jun 11;19(6):e06624. doi: 10.2903/j.efsa.2021.6624. eCollection 2021 Jun. EFSA J. 2021. PMID: 34136000 Free PMC article.
-
Safety and efficacy of a feed additive consisting of an essential oil from the fruits of Litsea cubeba (Lour.) Pers. (litsea berry oil) for use in all animal species (FEFANA asbl).EFSA J. 2021 Jun 11;19(6):e06623. doi: 10.2903/j.efsa.2021.6623. eCollection 2021 Jun. EFSA J. 2021. PMID: 34135999 Free PMC article.
-
Safety and efficacy of a feed additive consisting of an extract of condensed tannins from Schinopsis balansae Engl. and Schinopsis lorentzii (Griseb.) Engl. (red quebracho extract) for use in all animal species (FEFANA asbl).EFSA J. 2022 Dec 15;20(12):e07699. doi: 10.2903/j.efsa.2022.7699. eCollection 2022 Dec. EFSA J. 2022. PMID: 36540775 Free PMC article.
-
Safety and efficacy of a feed additive consisting of expressed mandarin oil from the fruit peels of Citrus reticulata Blanco for use in all animal species (FEFANA asbl).EFSA J. 2021 Jun 10;19(6):e06625. doi: 10.2903/j.efsa.2021.6625. eCollection 2021 Jun. EFSA J. 2021. PMID: 34136001 Free PMC article.
-
Safety and efficacy of feed additives consisting of expressed sweet orange peel oil and its fractions from Citrus sinensis (L.) Osbeck for use in all animal species (FEFANA asbl).EFSA J. 2021 Nov 19;19(11):e06891. doi: 10.2903/j.efsa.2021.6891. eCollection 2021 Nov. EFSA J. 2021. PMID: 34824643 Free PMC article.
Cited by
-
Assessment of the feed additive consisting of naringin for all animal species for the renewal of its authorisation (HealthTech Bio Actives, S.L.U. (HTBA)).EFSA J. 2022 Apr 22;20(4):e07267. doi: 10.2903/j.efsa.2022.7267. eCollection 2022 Apr. EFSA J. 2022. PMID: 35615734 Free PMC article.
-
Risk Assessment of Combined Exposure to Multiple Chemicals at the European Food Safety Authority: Principles, Guidance Documents, Applications and Future Challenges.Toxins (Basel). 2023 Jan 4;15(1):40. doi: 10.3390/toxins15010040. Toxins (Basel). 2023. PMID: 36668860 Free PMC article. Review.
-
Uroprotective and pain-relieving effect of dietary supplementation with micronized palmitoyl-glucosamine and hesperidin in a chronic model of cyclophosphamide-induced cystitis.Front Vet Sci. 2024 Jan 4;10:1327102. doi: 10.3389/fvets.2023.1327102. eCollection 2023. Front Vet Sci. 2024. PMID: 38249555 Free PMC article.
-
A Systematic Review: Quercetin-Secondary Metabolite of the Flavonol Class, with Multiple Health Benefits and Low Bioavailability.Int J Mol Sci. 2024 Nov 11;25(22):12091. doi: 10.3390/ijms252212091. Int J Mol Sci. 2024. PMID: 39596162 Free PMC article.
-
Safety and efficacy of feed additives obtained from the fruit of Pimpinella anisum L.: anise oil for use in poultry and horses and anise tincture for use in poultry, dogs, cats and horses (FEFANA asbl).EFSA J. 2023 Apr 20;21(4):e07976. doi: 10.2903/j.efsa.2023.7976. eCollection 2023 Apr. EFSA J. 2023. PMID: 37089173 Free PMC article.
References
-
- Bampidis V and Robinson PH, 2006. Citrus by‐products as ruminant feeds: a review. Animal Feed Science and Technology, 128, 175–217.
-
- Bang SH, Hyun YJ, Shim J, Hong SW and Kim DY, 2015. Metabolism of rutin and poncirin by human intestinal microbiota and cloning of their metabolizing α‐L‐rhamnosidase from bifidobacterium dentium. Journal of Microbiology and Biotechnology, 25, 18–25. - PubMed
-
- BfR (Bundesinstitut für Riskobewertung), 2012. Health assessment of sports and weight loss products containing synephrine and caffeine. BfR Opinion No. 004/2013, of 16 November 2012. Available online: https://mobil.bfr.bund.de/cm/349/health-assessment-of-sports-and-weight-...
-
- Bracher F, Heisig P, Langguth P, Mutschler E, Rücker G, Schirmeister T, Scriba G, Stahl‐Biskup E and Troschütz R, 2016. Kommentar zum Europäischen Arzneibuch (Commentary to the Pharmacopoeia Europaea). Wissenschaftliche Verlagsgesellschaft, Stuttgart, Germany.
LinkOut - more resources
Full Text Sources
Miscellaneous
