E2F4 Promotes the Proliferation of Hepatocellular Carcinoma Cells through Upregulation of CDCA3
- PMID: 34335934
- PMCID: PMC8317516
- DOI: 10.7150/jca.53708
E2F4 Promotes the Proliferation of Hepatocellular Carcinoma Cells through Upregulation of CDCA3
Abstract
Liver cancer, the second most commonly diagnosed cancer, is associated with high mortality rates. E2F4 is a member of the E2F transcription factor family. There are limited studies on the role of E2F4 in hepatocellular carcinoma (HCC). In this study, the expression of E2F4 in HCC tissue samples and cell lines was analyzed using quantitative real-time polymerase chain reaction. E2F4 expression positively correlated with tumor size in patients with HCC. Additionally, E2F4 expression was greater in HCC cells than in normal LO2 cells. Furthermore, overexpression of E2F4 significantly enhanced the proliferation, migration, and invasion of HCC cells. The results of a luciferase assay revealed that E2F4 upregulated the expression of CDCA3 by binding to its promoter region (1863'-ACGCGCGAGAATG-1875') and consequently promoted proliferation and cell cycle progression of HCC cells. Taken together, these results demonstrated that E2F4 might play a vital role in HCC progression and could serve as a potential biomarker for the diagnosis and as a therapeutic target of HCC.
Keywords: CDCA3; E2F4; hepatocellular carcinoma; promoter.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
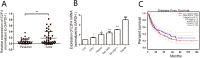



References
-
- Bray F, Ferlay J, Soerjomataram I. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. - PubMed
-
- Marrero JA, Kulik LM, Sirlin CB. et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–750. - PubMed
-
- Dimova DK, Dyson NJ. The E2F transcriptional network: old acquaintances with new faces. Oncogene. 2005;24:2810–2826. - PubMed
LinkOut - more resources
Full Text Sources

