Metronomic vinorelbine is an excellent and safe treatment for advanced breast cancer: a retrospective, observational study
- PMID: 34335952
- PMCID: PMC8317530
- DOI: 10.7150/jca.60682
Metronomic vinorelbine is an excellent and safe treatment for advanced breast cancer: a retrospective, observational study
Abstract
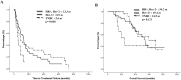
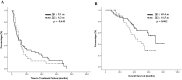
Advanced breast cancer (ABC) has become a chronic disease. In such a situation, an effective therapy with low toxicities and economically acceptable is needed. Metronomic vinorelbine (mVNR) has been proved to be effective on the control of MBC. The aim of this study is to evaluate the efficacy and safety of mVNR as the salvage therapy for patients with ABC. Oral vinorelbine (VNR) was administered at 70 mg/m2, fractionated on days 1, 3, and 5, for 3 weeks on and 1 week off. Once the mVNR was combined with trastuzumab, or was combined with bevacizumab, the schedule was changed to 2 weeks on and 1 week off. Clinical data of patients with ABC who had received treatment with mVNR and tumor characteristics were collected and analyzed. From Mar. 2013 to Dec, 2020, there were 90 patients with ABC received mVNR. The overall response rate was 53.3% and overall disease control rate (DCR) was 78.9% in this study, including 4 (4.4%) cases reached complete response, 44 (48.9%) cases reached partial response and 23 (25.6%) cases were table disease. The median time to treatment failure (TTF) of the Lumina A patients was 13.3 months, Lumina B patients was 9.1 months, Her-2 enrich patients was 8.9 months, and triple negative breast cancer (TNBC) patients was 5.6 months. Median overall survival time for Lumina A, Lumina B, Her-2 enrich and TNBC were 54.6 months, 53.3 months, 59.5 months and 24.5 months separately. Side effects were minimal and manageable. Metronomic VNR can be an effective treatment for ABC either works as a switch maintenance or salvage therapy. In combination with target therapy or hormonal therapy, mVNR can further improve TTF and DCR with minimal toxicities. Further study should focus on the optimal dosage, schedule and combination regimen.
Keywords: advanced breast cancer; effect.; metastatic breast cancer; metronomic chemotherapy; vinorelbine.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

