A perspective on the radiopharmaceutical requirements for imaging and therapy of glioblastoma
- PMID: 34335972
- PMCID: PMC8315062
- DOI: 10.7150/thno.56639
A perspective on the radiopharmaceutical requirements for imaging and therapy of glioblastoma
Abstract
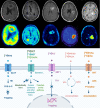
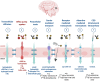
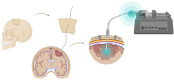
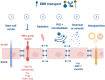
Despite numerous clinical trials and pre-clinical developments, the treatment of glioblastoma (GB) remains a challenge. The current survival rate of GB averages one year, even with an optimal standard of care. However, the future promises efficient patient-tailored treatments, including targeted radionuclide therapy (TRT). Advances in radiopharmaceutical development have unlocked the possibility to assess disease at the molecular level allowing individual diagnosis. This leads to the possibility of choosing a tailored, targeted approach for therapeutic modalities. Therapeutic modalities based on radiopharmaceuticals are an exciting development with great potential to promote a personalised approach to medicine. However, an effective targeted radionuclide therapy (TRT) for the treatment of GB entails caveats and requisites. This review provides an overview of existing nuclear imaging and TRT strategies for GB. A critical discussion of the optimal characteristics for new GB targeting therapeutic radiopharmaceuticals and clinical indications are provided. Considerations for target selection are discussed, i.e. specific presence of the target, expression level and pharmacological access to the target, with particular attention to blood-brain barrier crossing. An overview of the most promising radionuclides is given along with a validation of the relevant radiopharmaceuticals and theranostic agents (based on small molecules, peptides and monoclonal antibodies). Moreover, toxicity issues and safety pharmacology aspects will be presented, both in general and for the brain in particular.
Keywords: PET SPECT imaging; glioblastoma; radiochemistry; targeted radionuclide therapy; theranostics.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
-
- Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: A clinical review. J Am Med Assoc. 2013;310:1842–50. - PubMed
-
- Maher EA, Bachoo RM. Glioblastoma. Rosenberg's molecular and genetic basis of neurological and psychiatric disease. Fifth Edition. Elsevier Inc. 2014.
-
- Weller M, Weber RG, Willscher E, Vera Riehmer V, Hentschel B, Kreuz M. et al. Molecular classification of diffuse cerebral WHO grade II/III gliomas using genome- and transcriptome-wide profiling improves stratification of prognostically distinct patient groups. Acta Neuropathol. 2015;129:679–93. - PubMed
-
- Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK. et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

