Ablation of lncRNA Miat attenuates pathological hypertrophy and heart failure
- PMID: 34335976
- PMCID: PMC8315059
- DOI: 10.7150/thno.50990
Ablation of lncRNA Miat attenuates pathological hypertrophy and heart failure
Abstract
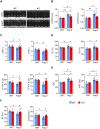
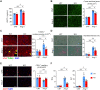
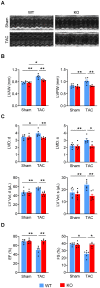
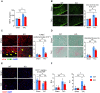
Rationale: The conserved long non-coding RNA (lncRNA) myocardial infarction associate transcript (Miat) was identified for its multiple single-nucleotide polymorphisms that are strongly associated with susceptibility to MI, but its role in cardiovascular biology remains elusive. Here we investigated whether Miat regulates cardiac response to pathological hypertrophic stimuli. Methods: Both an angiotensin II (Ang II) infusion model and a transverse aortic constriction (TAC) model were used in adult WT and Miat-null knockout (Miat-KO) mice to induce pathological cardiac hypertrophy. Heart structure and function were evaluated by echocardiography and histological assessments. Gene expression in the heart was evaluated by RNA sequencing (RNA-seq), quantitative real-time RT-PCR (qRT-PCR), and Western blotting. Primary WT and Miat-KO mouse cardiomyocytes were isolated and used in Ca2+ transient and contractility measurements. Results: Continuous Ang II infusion for 4 weeks induced concentric hypertrophy in WT mice, but to a lesser extent in Miat-KO mice. Surgical TAC for 6 weeks resulted in decreased systolic function and heart failure in WT mice but not in Miat-KO mice. In both models, Miat-KO mice displayed reduced heart-weight to tibia-length ratio, cardiomyocyte cross-sectional area, cardiomyocyte apoptosis, and cardiac interstitial fibrosis and a better-preserved capillary density, as compared to WT mice. In addition, Ang II treatment led to significantly reduced mRNA and protein expression of the Ca2+ cycling genes Sarcoplasmic/endoplasmic reticulum Ca2+ ATPase 2a (SERCA2a) and ryanodine receptor 2 (RyR2) and a dramatic increase in global RNA splicing events in the left ventricle (LV) of WT mice, and these changes were largely blunted in Miat-KO mice. Consistently, cardiomyocytes isolated from Miat-KO mice demonstrated more efficient Ca2+ cycling and greater contractility. Conclusions: Ablation of Miat attenuates pathological hypertrophy and heart failure, in part, by enhancing cardiomyocyte contractility.
Keywords: Miat; RNA splicing; cardiac hypertrophy; cardiomyocytes; heart failure; lncRNA.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

Similar articles
-
Long Non-Coding RNA Malat-1 Is Dispensable during Pressure Overload-Induced Cardiac Remodeling and Failure in Mice.PLoS One. 2016 Feb 26;11(2):e0150236. doi: 10.1371/journal.pone.0150236. eCollection 2016. PLoS One. 2016. PMID: 26919721 Free PMC article.
-
USP20 deletion promotes eccentric cardiac remodeling in response to pressure overload and increases mortality.Am J Physiol Heart Circ Physiol. 2024 Nov 1;327(5):H1257-H1271. doi: 10.1152/ajpheart.00329.2024. Epub 2024 Oct 4. Am J Physiol Heart Circ Physiol. 2024. PMID: 39365672
-
LncRNA MIAT enhances cardiac hypertrophy partly through sponging miR-150.Eur Rev Med Pharmacol Sci. 2016 Sep;20(17):3653-60. Eur Rev Med Pharmacol Sci. 2016. PMID: 27649667
-
Long non-coding RNA MIAT in development and disease: a new player in an old game.J Biomed Sci. 2018 Mar 13;25(1):23. doi: 10.1186/s12929-018-0427-3. J Biomed Sci. 2018. PMID: 29534728 Free PMC article. Review.
-
MIAT LncRNA: A multifunctional key player in non-oncological pathological conditions.Noncoding RNA Res. 2024 Jan 20;9(2):447-462. doi: 10.1016/j.ncrna.2024.01.011. eCollection 2024 Jun. Noncoding RNA Res. 2024. PMID: 38511054 Free PMC article. Review.
Cited by
-
Aerobic Exercise Inhibited P2X7 Purinergic Receptors to Improve Cardiac Remodeling in Mice With Type 2 Diabetes.Front Physiol. 2022 May 31;13:828020. doi: 10.3389/fphys.2022.828020. eCollection 2022. Front Physiol. 2022. PMID: 35711309 Free PMC article.
-
Astragaloside IV derivative HHQ16 ameliorates infarction-induced hypertrophy and heart failure through degradation of lncRNA4012/9456.Signal Transduct Target Ther. 2023 Oct 19;8(1):414. doi: 10.1038/s41392-023-01660-9. Signal Transduct Target Ther. 2023. PMID: 37857609 Free PMC article.
-
Long Non-coding RNA Involved in the Pathophysiology of Atrial Fibrillation.Cardiovasc Drugs Ther. 2025 Apr;39(2):435-458. doi: 10.1007/s10557-023-07491-8. Epub 2023 Sep 13. Cardiovasc Drugs Ther. 2025. PMID: 37702834 Free PMC article. Review.
-
Non-Coding RNAs in the Therapeutic Landscape of Pathological Cardiac Hypertrophy.Cells. 2022 May 31;11(11):1805. doi: 10.3390/cells11111805. Cells. 2022. PMID: 35681500 Free PMC article. Review.
-
The lncRNA MIAT regulates CPT-1a mediated cardiac hypertrophy through m6A RNA methylation reading protein Ythdf2.Cell Death Discov. 2022 Apr 5;8(1):167. doi: 10.1038/s41420-022-00977-8. Cell Death Discov. 2022. PMID: 35383152 Free PMC article.
References
-
- Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP. et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141:e139–e596. - PubMed
-
- Hill JA, Olson EN. Cardiac plasticity. The New England journal of medicine. 2008;358:1370–80. - PubMed
-
- Tham YK, Bernardo BC, Ooi JY, Weeks KL, McMullen JR. Pathophysiology of cardiac hypertrophy and heart failure: signaling pathways and novel therapeutic targets. Archives of toxicology. 2015;89:1401–38. - PubMed
-
- Nakamura M, Sadoshima J. Mechanisms of physiological and pathological cardiac hypertrophy. Nature reviews Cardiology. 2018;15:387–407. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

