False-Positive Maternal Serum Screens in the Second Trimester as Markers of Placentally Mediated Complications Later in Pregnancy: A Systematic Review and Meta-Analysis
- PMID: 34336005
- PMCID: PMC8295507
- DOI: 10.1155/2021/5566234
False-Positive Maternal Serum Screens in the Second Trimester as Markers of Placentally Mediated Complications Later in Pregnancy: A Systematic Review and Meta-Analysis
Abstract
Background: Multiple-marker, maternal serum screening (MSS) has been the cornerstone of prenatal diagnosis since the 1980s. While combinations of these markers are used to predict fetal risk of Down syndrome and other genetic conditions, there is some evidence that individual markers may also predict nongenetic pregnancy complications, particularly those related to placental dysfunction. The objective of this meta-analysis was to investigate the utility of false-positive, second-trimester MSS for Down syndrome as a marker of placentally mediated complications amongst singleton pregnancies globally.
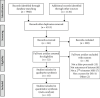
Methods: Electronic searches of PubMed, Medline, Embase, CINAHL, Web of Science, Scopus, and grey literature to 2019 were performed to identify observational studies comparing risk of pregnancy complications amongst pregnancies with false-positive MSS versus controls. A random-effects model of pooled odds ratios by outcome of interest (stillbirth, preeclampsia, fetal growth restriction, and preterm birth) and subgrouped by type of MSS test (double-, triple-, and quadruple-marker MSS) was used.
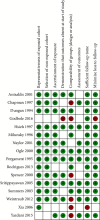
Results: 16 studies enrolling 68515 pregnancies were included. There were increased odds of preeclampsia (OR 1.28, 95% CI 1.09-1.51) and stillbirth (OR 2.46, 95% CI 1.94-3.12) amongst pregnancies with false-positive MSS. There was no significant association with preterm birth or growth restriction.
Conclusions: There is some evidence of an association between false-positive, second-trimester MSS for Down syndrome and increased odds of preeclampsia and stillbirth. Future large-scale prospective studies are still needed to best determine the predictive value of false-positive MSS as a marker of placentally mediated complications later in pregnancy and evaluate potential clinical interventions to reduce these risks.
Copyright © 2021 Christy L. Pylypjuk and Joel Monarrez-Espino.
Conflict of interest statement
The authors deny any conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

