Pathogenesis of autoimmune demyelination: from multiple sclerosis to neuromyelitis optica spectrum disorders and myelin oligodendrocyte glycoprotein antibody-associated disease
- PMID: 34336206
- PMCID: PMC8312887
- DOI: 10.1002/cti2.1316
Pathogenesis of autoimmune demyelination: from multiple sclerosis to neuromyelitis optica spectrum disorders and myelin oligodendrocyte glycoprotein antibody-associated disease
Abstract
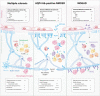
Autoimmunity plays a significant role in the pathogenesis of demyelination. Multiple sclerosis (MS), neuromyelitis optica spectrum disorders (NMOSD) and myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD) are now recognised as separate disease entities under the amalgam of human central nervous system demyelinating disorders. While these disorders share inherent similarities, investigations into their distinct clinical presentations and lesion pathologies have aided in differential diagnoses and understanding of disease pathogenesis. An interplay of various genetic and environmental factors contributes to each disease, many of which implicate an autoimmune response. The pivotal role of the adaptive immune system has been highlighted by the diagnostic autoantibodies in NMOSD and MOGAD, and the presence of autoreactive lymphocytes in MS lesions. While a number of autoantigens have been proposed in MS, recent emphasis on the contribution of B cells has shed new light on the well-established understanding of T cell involvement in pathogenesis. This review aims to synthesise the clinical characteristics and pathological findings, discuss existing and emerging hypotheses regarding the aetiology of demyelination and evaluate recent pathogenicity studies involving T cells, B cells, and autoantibodies and their implications in human demyelination.
Keywords: AQP4 antibody; MOG antibody; autoimmune demyelination; multiple sclerosis; neuromyelitis optica spectrum disorders; pathology.
© 2021 The Authors. Clinical & Translational Immunology published by John Wiley & Sons Australia, Ltd on behalf of Australian and New Zealand Society for Immunology, Inc.
Conflict of interest statement
JAL reports funding from the Research Training Program Scholarship (Australia). SR has received competitive research funding from the National Health and Medical Research Council (Australia), the Petre Foundation (Australia), the Brain Foundation (Australia), the Royal Australasian College of Physicians and the University of Sydney; and is currently supported by an NHMRC Early Career Fellowship (APP1141169). SR is a consultant on an advisory board for UCB and Limbic Neurology and has received honoraria from Biogen and Limbic Neurology as an invited speaker. RCD and FB have received research funding from The Trish Multiple Sclerosis Research Foundation, Multiple Sclerosis Research Australia, the Petre Foundation and the National Health Medical Research Council (Australia). They have received honoraria from Biogen Idec and Merck as invited speakers. MD declares no competing interests.
Figures
References
-
- Inusah S, Sormani MP, Cofield SS et al. Assessing changes in relapse rates in multiple sclerosis. Mult Scler J 2010; 16: 1414–1421. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
