A Comparative Study of Ranibizumab and Aflibercept for Neovascular Age-Related Macular Degeneration: 12-Month Outcomes of Polish Therapeutic Program in Non-Tertiary Institution
- PMID: 34336421
- PMCID: PMC8312781
- DOI: 10.7759/cureus.15916
A Comparative Study of Ranibizumab and Aflibercept for Neovascular Age-Related Macular Degeneration: 12-Month Outcomes of Polish Therapeutic Program in Non-Tertiary Institution
Abstract
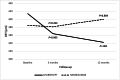
Introduction This single-center study aimed to compare the 12-month treatment outcomes of ranibizumab with that of aflibercept in routine clinical practice. Methods Cohort of patients diagnosed with treatment-naïve neovascular age-related macular degeneration (AMD), treated using either ranibizumab (n = 33 eyes) or aflibercept (n = 44 eyes) monotherapy over a 12-month follow-up period was analyzed. Anonymous data were extracted from the electronic database dedicated to the drug program. Results In the ranibizumab group, there were no statistically significant changes in best-corrected visual acuity (BCVA) (Early Treatment Diabetic Retinopathy Study [ETDRS] letters) and central retina thickness (CRT) (µm), between baseline (67.9 ± 8.6 & 384.9 ± 97.9) and at 12 months (67.9 ± 12.1 & 398.9 ± 127.1; P = 0.372 & P = 0.884, respectively). In the aflibercept, there was an improvement in BCVA and reduction in CRT between baseline (64.2 ± 8.1 & 414.3 ± 97.8) and at 12 months (70.7 ± 7.4 & 342.3 ± 71.6; P < 0.001 & P < 0.001, respectively). There was no difference in BCVA between the two groups at either diagnosis (P = 0.101) or 12 months (P = 0.917). Mean number of injections in the ranibizumab group was significantly lower (4.9 ± 1.5) than in the aflibercept group (6.7 ± 1; P < 0.001). Conclusions One initial injection of ranibizumab and then pro re nata (PRN) regimen resulted in stabilization of disease progression. Drug selection and treatment scheme could influence twelve-months outcomes. In the aflibercept group, three initial monthly injections and then every two months provided both significant BCVA improvement and CRT reduction at 12 months of treatment.
Keywords: aflibercept; aflibercept intravitreal injection; amd; ranibizumab; ranibizumab intravitreal injection; therapeutic program.
Copyright © 2021, Skrzypczak et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Current advances in the treatment of neovascular age-related macular degeneration. Villegas VM, Aranguren LA, Kovach JL, Schwartz SG, Flynn HW Jr. Expert Opin Drug Deliv. 2017;14:273–282. - PubMed
-
- One year outcomes of wet age-related macular degeneration treatment with aflibercept in therapeutic program. Figurska M. http://semanticscholar.org/paper/One-year-outcomes-of-wet-age-related-ma... Ocena Rocz. Ef. Ter. 2020;121:194.
LinkOut - more resources
Full Text Sources
Research Materials