Evaluation of a Prototype of a Novel Galactomannan Sandwich Assay Using the VIDAS® Technology for the Diagnosis of Invasive Aspergillosis
- PMID: 34336710
- PMCID: PMC8322699
- DOI: 10.3389/fcimb.2021.669237
Evaluation of a Prototype of a Novel Galactomannan Sandwich Assay Using the VIDAS® Technology for the Diagnosis of Invasive Aspergillosis
Abstract
Objectives: To evaluate the analytical and clinical performance of a prototype of a VIDAS® Galactomannan (GM) unitary test (bioMérieux, Marcy l'Etoile, France) and compare to that of the Platelia™ Aspergillus Ag assay (Bio-Rad, CA, USA).
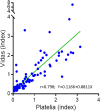
Methods: Repeatability, reproducibility, and freeze-thaw stability of VIDAS®GM were evaluated. Sera from patients at risk of IA were concurrently tested with both the VIDAS®GM and Platelia™ Aspergillus Ag assays. Correlations between the two assays were assessed by Passing Bablok (PB) regression and performance by ROC analysis.
Results: The correlations between the VIDAS®GM indexes after one and two cycles of freezing/thawing were r=1.00 and r=0.989, respectively. The coefficients of variation for negative, low-positive, and positive sera were 13%, 6%, and 5% for repeatability and 14.4%, 7.2%, and 5.5% for reproducibility. Overall, 126 sera were tested with both assays (44 fresh and 82 frozen). The correlation between VIDAS®GM and Platelia™ Aspergillus Ag was r=0.798. The areas under the curve of the ROC analyses were 0.892 and 0.894, for VIDAS®GM and Platelia™ Aspergillus Ag, respectively.
Conclusions: This new VIDAS®GM prototype assay showed adequate analytical and clinical performance and a good correlation with that of Platelia™ Aspergillus Ag with 126 sera, although these results need to be confirmed in a larger prospective and multicentric study. As for the other VIDAS® assays, VIDAS®GM is a single-sample automated test using a solid reagent strip and receptacle. It is easy to use and suitable for rapid on-demand test results.
Keywords: VIDAS®; diagnosis; galactomannan; invasive aspergillosis; single-sample test.
Copyright © 2021 Gallet, Garnaud, Dragonetti, Rivoiron, Cognet, Guo, Lesénéchal, Maubon and Cornet.
Conflict of interest statement
CD, SR, YG, and ML are bioMérieux employees. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer FG declared a past co-authorship with several of the authors DM, MC, to the handling Editor.
Figures

References
-
- Donnelly J. P., Chen S. C., Kauffman C. A., Steinbach W. J., Baddley J. W., Verweij P. E., et al. . (2020). Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 71, 1367–1376. 10.1093/cid/ciz1008 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

