Tissue Factor-Enriched Neutrophil Extracellular Traps Promote Immunothrombosis and Disease Progression in Sepsis-Induced Lung Injury
- PMID: 34336711
- PMCID: PMC8317465
- DOI: 10.3389/fcimb.2021.677902
Tissue Factor-Enriched Neutrophil Extracellular Traps Promote Immunothrombosis and Disease Progression in Sepsis-Induced Lung Injury
Abstract
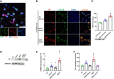
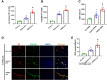
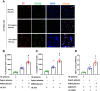
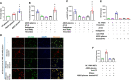
Background: Patients with sepsis may progress to acute respiratory distress syndrome (ARDS). Evidence of neutrophil extracellular traps (NETs) in sepsis-induced lung injury has been reported. However, the role of circulating NETs in the progression and thrombotic tendency of sepsis-induced lung injury remains elusive. The aim of this study was to investigate the role of tissue factor-enriched NETs in the progression and immunothrombosis of sepsis-induced lung injury.
Methods: Human blood samples and an animal model of sepsis-induced lung injury were used to detect and evaluate NET formation in ARDS patients. Immunofluorescence imaging, ELISA, Western blotting, and qPCR were performed to evaluate in vitro NET formation and tissue factor (TF) delivery ability. DNase, an anti-TF antibody, and thrombin inhibitors were applied to evaluate the contribution of thrombin to TF-enriched NET formation and the contribution of TF-enriched NETs to immunothrombosis in ARDS patients.
Results: Significantly increased levels of TF-enriched NETs were observed in ARDS patients and mice. Blockade of NETs in ARDS mice alleviated disease progression, indicating a reduced lung wet/dry ratio and PaO2 level. In vitro data demonstrated that thrombin-activated platelets were responsible for increased NET formation and related TF exposure and subsequent immunothrombosis in ARDS patients.
Conclusion: The interaction of thrombin-activated platelets with PMNs in ARDS patients results in local NET formation and delivery of active TF. The notion that NETs represent a mechanism by which PMNs release thrombogenic signals during thrombosis may offer novel therapeutic targets.
Keywords: acute lung injury; immunothrombosis; neutrophil extracellular traps; sepsis; tissue factor.
Copyright © 2021 Zhang, Zhou, Qu, Yu, Chen, Zhu, Guo, Chen and Miao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Expression of functional tissue factor by neutrophil extracellular traps in culprit artery of acute myocardial infarction.Eur Heart J. 2015 Jun 7;36(22):1405-14. doi: 10.1093/eurheartj/ehv007. Epub 2015 Feb 7. Eur Heart J. 2015. PMID: 25660055 Free PMC article.
-
Glycyrrhizin alleviates sepsis-induced acute respiratory distress syndrome via suppressing of HMGB1/TLR9 pathways and neutrophils extracellular traps formation.Int Immunopharmacol. 2022 Jul;108:108730. doi: 10.1016/j.intimp.2022.108730. Epub 2022 Mar 24. Int Immunopharmacol. 2022. PMID: 35354111
-
Maladaptive role of neutrophil extracellular traps in pathogen-induced lung injury.JCI Insight. 2018 Feb 8;3(3):e98178. doi: 10.1172/jci.insight.98178. eCollection 2018 Feb 8. JCI Insight. 2018. PMID: 29415887 Free PMC article.
-
Role and intervention of PAD4 in NETs in acute respiratory distress syndrome.Respir Res. 2024 Jan 30;25(1):63. doi: 10.1186/s12931-024-02676-7. Respir Res. 2024. PMID: 38291476 Free PMC article. Review.
-
Review: The Emerging Role of Neutrophil Extracellular Traps in Sepsis and Sepsis-Associated Thrombosis.Front Cell Infect Microbiol. 2021 Mar 17;11:653228. doi: 10.3389/fcimb.2021.653228. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33816356 Free PMC article. Review.
Cited by
-
Neutrophil extracellular traps contribute to immunothrombosis formation via the STING pathway in sepsis-associated lung injury.Cell Death Discov. 2023 Aug 25;9(1):315. doi: 10.1038/s41420-023-01614-8. Cell Death Discov. 2023. PMID: 37626060 Free PMC article.
-
Neutrophils: Linking Inflammation to Thrombosis and Unlocking New Treatment Horizons.Int J Mol Sci. 2025 Feb 25;26(5):1965. doi: 10.3390/ijms26051965. Int J Mol Sci. 2025. PMID: 40076593 Free PMC article. Review.
-
Friend or foe: the role of platelets in acute lung injury.Front Immunol. 2025 May 14;16:1556923. doi: 10.3389/fimmu.2025.1556923. eCollection 2025. Front Immunol. 2025. PMID: 40438116 Free PMC article. Review.
-
Analysis of the effects of early screening combined with blood lactate on the severity of patients with sepsis.Heliyon. 2024 May 23;10(11):e31907. doi: 10.1016/j.heliyon.2024.e31907. eCollection 2024 Jun 15. Heliyon. 2024. PMID: 38947447 Free PMC article.
-
Neutrophil Extracellular Traps and Respiratory Disease.J Clin Med. 2024 Apr 19;13(8):2390. doi: 10.3390/jcm13082390. J Clin Med. 2024. PMID: 38673662 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

