SCA-1/Ly6A Mesodermal Skeletal Progenitor Subpopulations Reveal Differential Commitment of Early Limb Bud Cells
- PMID: 34336823
- PMCID: PMC8322737
- DOI: 10.3389/fcell.2021.656999
SCA-1/Ly6A Mesodermal Skeletal Progenitor Subpopulations Reveal Differential Commitment of Early Limb Bud Cells
Abstract
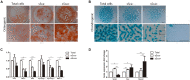
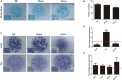
At early developmental stages, limb bud mesodermal undifferentiated cells are morphologically indistinguishable. Although the identification of several mesodermal skeletal progenitor cell populations has been recognized, in advanced stages of limb development here we identified and characterized the differentiation hierarchy of two new early limb bud subpopulations of skeletal progenitors defined by the differential expression of the SCA-1 marker. Based on tissue localization of the mesenchymal stromal cell-associated markers (MSC-am) CD29, Sca-1, CD44, CD105, CD90, and CD73, we identified, by multiparametric analysis, the presence of cell subpopulations in the limb bud capable of responding to inductive signals differentially, namely, sSca+ and sSca- cells. In concordance with its gene expression profile, cell cultures of the sSca+ subpopulation showed higher osteogenic but lower chondrogenic capacity than those of sSca-. Interestingly, under high-density conditions, fibroblast-like cells in the sSca+ subpopulation were abundant. Gain-of-function employing micromass cultures and the recombinant limb assay showed that SCA-1 expression promoted tenogenic differentiation, whereas chondrogenesis is delayed. This model represents a system to determine cell differentiation and morphogenesis of different cell subpopulations in similar conditions like in vivo. Our results suggest that the limb bud is composed of a heterogeneous population of progenitors that respond differently to local differentiation inductive signals in the early stages of development, where SCA-1 expression may play a permissive role during cell fate.
Keywords: SCA-1/Ly6A; chondrogenesis; limb bud; progenitor cell; recombinant limbs; tenogenic differentiation.
Copyright © 2021 Marín-Llera, Lorda-Diez, Hurle and Chimal-Monroy.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Commitment of human mesenchymal stromal cells to skeletal lineages is independent of their morphogenetic capacity.World J Stem Cells. 2023 Jul 26;15(7):701-712. doi: 10.4252/wjsc.v15.i7.701. World J Stem Cells. 2023. PMID: 37545756 Free PMC article.
-
A small population of resident limb bud mesenchymal cells express few MSC-associated markers, but the expression of these markers is increased immediately after cell culture.Cell Biol Int. 2018 May;42(5):570-579. doi: 10.1002/cbin.10933. Epub 2018 Feb 1. Cell Biol Int. 2018. PMID: 29314362
-
Ontogenic Identification and Analysis of Mesenchymal Stromal Cell Populations during Mouse Limb and Long Bone Development.Stem Cell Reports. 2017 Oct 10;9(4):1124-1138. doi: 10.1016/j.stemcr.2017.08.007. Epub 2017 Sep 14. Stem Cell Reports. 2017. PMID: 28919259 Free PMC article.
-
The recombinant limb as a model for the study of limb patterning, and its application to muscle development.Cell Tissue Res. 1999 Apr;296(1):121-9. doi: 10.1007/s004410051273. Cell Tissue Res. 1999. PMID: 10199972 Review.
-
Ectoderm as a determinant of early tissue pattern in the limb bud.Cell Differ. 1984 Nov;15(1):17-24. doi: 10.1016/0045-6039(84)90025-3. Cell Differ. 1984. PMID: 6394145 Review.
Cited by
-
Aging-related changes of miR-23b-3p expression in extracellular vesicles from mesenchymal stromal cells affect TGFBR3 signaling.Sci Rep. 2025 Jul 1;15(1):22186. doi: 10.1038/s41598-025-06982-y. Sci Rep. 2025. PMID: 40596573 Free PMC article.
-
Recombinant Limb Assay as in Vivo Organoid Model.Front Cell Dev Biol. 2022 Apr 26;10:863140. doi: 10.3389/fcell.2022.863140. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35557939 Free PMC article.
-
Trajectory-centric framework TrajAtlas reveals multi-scale differentiation heterogeneity among cells, genes, and gene modules in osteogenesis.PLoS Genet. 2024 Oct 22;20(10):e1011319. doi: 10.1371/journal.pgen.1011319. eCollection 2024 Oct. PLoS Genet. 2024. PMID: 39436962 Free PMC article.
-
ZIM1 Combined with Hydrogel Inhibits Senescence of Primary PαS Cells during In Vitro Expansion.Int J Mol Sci. 2023 Jun 5;24(11):9766. doi: 10.3390/ijms24119766. Int J Mol Sci. 2023. PMID: 37298717 Free PMC article.
References
-
- Akiyama H., Chaboissier M.-C., Martin J. F., Schedl A., de Crombrugghe B. (2002). The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 16 2813–2828. 10.1101/gad.1017802 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

