Caspases in the Developing Central Nervous System: Apoptosis and Beyond
- PMID: 34336853
- PMCID: PMC8322698
- DOI: 10.3389/fcell.2021.702404
Caspases in the Developing Central Nervous System: Apoptosis and Beyond
Abstract
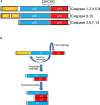
The caspase family of cysteine proteases represents the executioners of programmed cell death (PCD) type I or apoptosis. For years, caspases have been known for their critical roles in shaping embryonic structures, including the development of the central nervous system (CNS). Interestingly, recent findings have suggested that aside from their roles in eliminating unnecessary neural cells, caspases are also implicated in other neurodevelopmental processes such as axon guidance, synapse formation, axon pruning, and synaptic functions. These results raise the question as to how neurons regulate this decision-making, leading either to cell death or to proper development and differentiation. This review highlights current knowledge on apoptotic and non-apoptotic functions of caspases in the developing CNS. We also discuss the molecular factors involved in the regulation of caspase-mediated roles, emphasizing the mitochondrial pathway of cell death.
Keywords: apoptosis; caspases; central nervous system; embryonic development; mitochondria.
Copyright © 2021 Nguyen, Gillet and Popgeorgiev.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

