Effect of the Concentration and the Type of Dispersant on the Synthesis of Copper Oxide Nanoparticles and Their Potential Antimicrobial Applications
- PMID: 34337198
- PMCID: PMC8319940
- DOI: 10.1021/acsomega.1c00818
Effect of the Concentration and the Type of Dispersant on the Synthesis of Copper Oxide Nanoparticles and Their Potential Antimicrobial Applications
Abstract
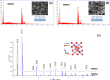
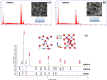
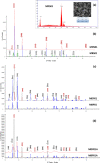
The bactericidal properties of copper oxide nanoparticles have growing interest due to potential application in the medical area. The present research investigates the influence of sodium dodecyl sulfate (SDS) and poly(vinylpyrrolidone) (PVP) on the production of copper oxide nanoparticles prepared from copper sulfate (CuSO4) and sodium borohydride (NaBH4) solutions. Different analytical techniques were used to determine the crystal nature, mean size diameter, and surface morphology of the copper oxide nanoparticles. The X-ray diffraction (XRD) patterns showed formation of nanoparticles of cuprite (Cu2O) and tenorite (CuO) when PVP and SDS were added at the beginning of the reaction. In fact, when the Cu/PVP ratio was 1.62, Cu2O nanoparticles were obtained. In addition, nanoparticles of CuO were synthesized when the Cu/PVP ratios were 0.54 and 0.81. On the other hand, a mixture of copper oxides (CuO and Cu2O) and cuprite (Cu2O) was obtained when PVP (Cu/PVP = 0.81 and 1.62) and SDS (Cu/SDS = 0.90) were added 30 min after the beginning of the reaction. Transmission electron microscopy (TEM) images show agglomerated nanoparticles with a size distribution ranging from 2 to 60 nm, while individual particles have sizes between 4.1 ± 1.9 and 41.6 ± 12.8 nm. The Kirby-Bauer method for the determination of antibacterial activity shows that small CuO (4.1 ± 1.9 nm) and Cu2O (8.5 ± 5.3 nm) nanoparticles inhibit the growth of Escherichia coli, Staphylococcus aureus MRSA, S. aureus and Pseudomonas aeruginosa bacteria. The antibacterial test of cotton fabric impregnated with nanoparticles shows positive results. The determination of the optimal ratio of copper oxide nanoparticles per cm2 of fabric that are able to exhibit a good antibacterial activity is ongoing.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Bai Y.; Yang T.; Gu Q.; Cheng G.; Zheng R. Shape control mechanism of cuprous oxide nanoparticles in aqueous colloidal solution. Powder Technol. 2012, 227, 35–42. 10.1016/j.powtec.2012.02.008. - DOI
-
- Ramyadevi J.; Jeyasubramanian K.; Marikani A.; Rajakumar G.; Rahuman A. A. Synthesis and antimicrobial activity of copper nanoparticles. Mater. Lett. 2012, 71, 114–116. 10.1016/j.matlet.2011.12.055. - DOI
- Pal S.; Tak Y. K.; Song J. M. Does the Antibacterial Activity of Silver Nanoparticles Depend on the Shape of the Nanoparticle? A Study of the Gram-Negative Bacterium Escherichia coli. Appl. Environ. Microbiol 2007, 73, 1712–1720. 10.1128/AEM.02218-06. - DOI - PMC - PubMed
-
- Jillani S.; Jelani M.; Hassan N. U.; Ahmad S.; Hafeez M. Synthesis, characterization and biological studies of copper oxide nanostructures. Mater. Res. Express 2018, 5, 04500610.1088/2053-1591/aab864. - DOI
-
- El-Nahhal I. M.; Elmanama A. A.; Amara N. A.. Synthesis of Nanometal Oxide–Coated Cotton Composites. In Cotton Research; Abdurakhmonov Y. I., Ed.; IntechOpen: London, 2016; pp 279–297.
- Rivero P. J.; Urrutia A.; Goicoechea J.; Arregui F. J. Nanomaterials for Functional Textiles and Fibers. Nanoscale Res Lett. 2015, 10, 50110.1186/s11671-015-1195-6. - DOI - PMC - PubMed
- Sedighi A.; Montazer M.; Samadi N. Synthesis of nano Cu2O on cotton: Morphological, physical, biological and optical sensing characterizations. Carbohydr. Polym. 2014, 110, 489–498. 10.1016/j.carbpol.2014.04.030. - DOI - PubMed
- El-Nahhal I. M.; Zourab S. M.; Kodeh F. S.; Selmane M.; Genois I.; Babonneau F. Nanostructured copper oxide-cotton fibers: synthesis, characterization, and applications. Int. Nano Lett. 2012, 2, 1410.1186/2228-5326-2-14. - DOI
-
- Khodashenas B.; Ghorbani H. R. Synthesis of copper nanoparticles: An overview of the various methods. Korean J. Chem. Eng. 2014, 31, 1105–1109. 10.1007/s11814-014-0127-y. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

