Effect of the Solvent and Substituent on Tautomeric Preferences of Amine-Adenine Tautomers
- PMID: 34337229
- PMCID: PMC8320138
- DOI: 10.1021/acsomega.1c02118
Effect of the Solvent and Substituent on Tautomeric Preferences of Amine-Adenine Tautomers
Abstract
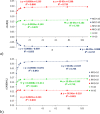
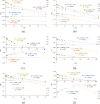
Adenine is one of the basic molecules of life; it is also an important building block in the synthesis of new pharmaceuticals, electrochemical (bio)sensors, or self-assembling molecular materials. Therefore, it is important to know the effects of the solvent and substituent on the electronic structure of adenine tautomers and their stability. The four most stable adenine amino tautomers (9H, 7H, 3H, and 1H), modified by substitution (C2- or C8-) of electron-withdrawing NO2 and electron-donating NH2 groups, are studied theoretically in the gas phase and in solvents of different polarities (1 ≤ ε < 109). Solvents have been modeled using the polarizable continuum model. Comparison of the stability of substituted adenine tautomers in various solvents shows that substitution can change tautomeric preferences with respect to the unsubstituted adenine. Moreover, C8 substitution results in slight energy differences between tautomers in polar solvents (<1 kcal/mol), which suggests that in aqueous solution, C8-X-substituted adenine systems may consist of a considerable amount of two tautomers-9H and 7H for X = NH2 and 3H and 9H for X = NO2. Furthermore, solvation enhances the effect of the nitro group; however, the enhancement strongly depends on the proximity effects. This enhancement for the NO2 group with two repulsive N···ON contacts can be threefold higher than that for the NO2 with one attractive NH···ON contact. The proximity effects are even more significant for the NH2 group, as the solvation may increase or decrease its electron-donating ability, depending on the type of proximity.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Influence of the Solvent on the Stability of Aminopurine Tautomers and Properties of the Amino Group.Molecules. 2023 Mar 27;28(7):2993. doi: 10.3390/molecules28072993. Molecules. 2023. PMID: 37049758 Free PMC article.
-
Intramolecular Interactions in Derivatives of Uracil Tautomers.Molecules. 2022 Oct 25;27(21):7240. doi: 10.3390/molecules27217240. Molecules. 2022. PMID: 36364066 Free PMC article.
-
Impact of the Substituents on the Electronic Structure of the Four Most Stable Tautomers of Purine and Their Adenine Analogues.ACS Omega. 2020 May 12;5(20):11570-11577. doi: 10.1021/acsomega.0c00820. eCollection 2020 May 26. ACS Omega. 2020. PMID: 32478247 Free PMC article.
-
Substituent effects on the stability of the four most stable tautomers of adenine and purine.RSC Adv. 2019 Oct 2;9(54):31343-31356. doi: 10.1039/c9ra04615a. eCollection 2019 Oct 1. RSC Adv. 2019. PMID: 35527924 Free PMC article.
-
Effect of H-bonding and complexation with metal ions on the π-electron structure of adenine tautomers.Org Biomol Chem. 2014 Jan 21;12(3):456-66. doi: 10.1039/c3ob41653d. Epub 2013 Nov 25. Org Biomol Chem. 2014. PMID: 24270549
Cited by
-
Influence of the Solvent on the Stability of Aminopurine Tautomers and Properties of the Amino Group.Molecules. 2023 Mar 27;28(7):2993. doi: 10.3390/molecules28072993. Molecules. 2023. PMID: 37049758 Free PMC article.
-
Intramolecular Interactions in Derivatives of Uracil Tautomers.Molecules. 2022 Oct 25;27(21):7240. doi: 10.3390/molecules27217240. Molecules. 2022. PMID: 36364066 Free PMC article.
-
Fabrication and evaluation of polylactic acid-curcumin containing carbon nanotubes (CNTs) wound dressing using electrospinning method with experimental and computational approaches.Sci Rep. 2025 Apr 18;15(1):13398. doi: 10.1038/s41598-025-98393-2. Sci Rep. 2025. PMID: 40251413 Free PMC article.
References
-
- Saenger W.Principles of Nucleic Acids Structures; Springer: New York, 1990.
-
- Pozharskii A. F.; Soldatenkov A. T.; Katritzky A. R.. Heterocycles in Life and Society; Wiley: New York, 1997.
LinkOut - more resources
Full Text Sources
Miscellaneous

