Effects of Solvents and pH Values on the Chemical Affinity of 10-Methacryloyloxydecyl Dihydrogen Phosphate toward Hydroxyapatite
- PMID: 34337256
- PMCID: PMC8320082
- DOI: 10.1021/acsomega.1c02521
Effects of Solvents and pH Values on the Chemical Affinity of 10-Methacryloyloxydecyl Dihydrogen Phosphate toward Hydroxyapatite
Abstract
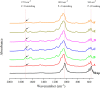
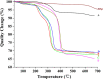
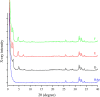
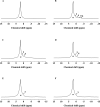
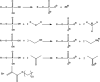
This study aimed to investigate the effects of solvents and pH values on the chemical interaction between 10-methacryloyloxydecyl dihydrogen phosphate (MDP) and hydroxyapatite (HAp). The chemical affinity of MDP toward HAp dissolved in different solvents (E-MDP: 10 wt % MDP and 90 wt % ethanol; E-W-MDP1: 10 wt % MDP, 75 wt % ethanol, and 15 wt % water; A-W-MDP: 10 wt % MDP, 75 wt % acetone, and 15 wt % water; and E-W-MDP2: 10 wt % MDP, 45 wt % ethanol, and 45 wt % water) was investigated. The pH of E-W-MDP2 was increased from 2.04 to 5 (E-W-MDP2/5) and to 7 (E-W-MDP2/7). The reaction products were characterized by Fourier transform infrared spectroscopy, X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), thermogravimetric analysis (TGA), and nuclear magnetic resonance (NMR). XRD and NMR results revealed that no MDP-calcium salt formed in E-MDP. XRD, TGA, and XPS results indicated that MDP interacted with HAp, producing the MDP-calcium salt in all groups except E-MDP. NMR results revealed that the dicalcium salt of the MDP dimer (DCS-MD) and the MDP tripolymer (DCS-MT) and the monocalcium salt of the MDP monomer and the MDP dimer were formed in E-W-MDP1. DCS-MD and DCS-MT were also formed in E-W-MDP2 and A-W-MDP. In E-W-MDP2/5 and E-W-MDP2/7, DCS-MD was obtained. Both the solvents and pH values affect the chemical interactions between MDP and HAp and the types of reaction products formed. MDP and HAp do not form any MDP-calcium salt in pure ethanol; the structural stability of MDP-calcium salts is dependent on the solvent water content and the pH value. The ethanol/water mixture is recommended as the main solvent in an MDP-containing primer, and the ideal pH value is 2-7; if these conditions are satisfied, sufficient amounts of MDP-calcium salts with stable structures are expected to be formed, thus improving the longevity of dentin/enamel bonding.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Van Landuyt K. L.; Snauwaert J.; De Munck J.; Peumans M.; Yoshida Y.; Poitevin A.; Coutinho E.; Suzuki K.; Lambrechts P.; Van Meerbeek B. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials 2007, 28, 3757–3785. 10.1016/j.biomaterials.2007.04.044. - DOI - PubMed
-
- Tian F.-c.; Wang X.-y.; Huang Q.; Niu L.-n.; Mitchell J.; Zhang Z.-y.; Prananik C.; Zhang L.; Chen J.-h.; Breshi L.; Pashley D. H.; Tay F. R. Effect of nanolayering of calcium salts of phosphoric acid ester monomers on the durability of resin-dentin bonds. Acta Biomater. 2016, 38, 190–200. 10.1016/j.actbio.2016.04.034. - DOI - PubMed
LinkOut - more resources
Full Text Sources

