A single cell transcriptomics map of paracrine networks in the intrinsic cardiac nervous system
- PMID: 34337356
- PMCID: PMC8324809
- DOI: 10.1016/j.isci.2021.102713
A single cell transcriptomics map of paracrine networks in the intrinsic cardiac nervous system
Abstract
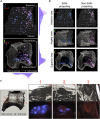
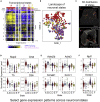
We developed a spatially-tracked single neuron transcriptomics map of an intrinsic cardiac ganglion, the right atrial ganglionic plexus (RAGP) that is a critical mediator of sinoatrial node (SAN) activity. This 3D representation of RAGP used neuronal tracing to extensively map the spatial distribution of the subset of neurons that project to the SAN. RNA-seq of laser capture microdissected neurons revealed a distinct composition of RAGP neurons compared to the central nervous system and a surprising finding that cholinergic and catecholaminergic markers are coexpressed, suggesting multipotential phenotypes that can drive neuroplasticity within RAGP. High-throughput qPCR of hundreds of laser capture microdissected single neurons confirmed these findings and revealed a high dimensionality of neuromodulatory factors that contribute to dynamic control of the heart. Neuropeptide-receptor coexpression analysis revealed a combinatorial paracrine neuromodulatory network within RAGP informing follow-on studies on the vagal control of RAGP to regulate cardiac function in health and disease.
Keywords: Cardiovascular medicine; Molecular physiology; Systems neuroscience; Transcriptomics.
© 2021 The Authors.
Conflict of interest statement
Competing interests: University of California, Los Angeles has patents developed by KS and JLA relating to cardiac neural diagnostics and therapeutics. KS and JLA are co-founders of NeuCures, Inc. All the remaining authors declare no competing interests.
Figures





References
-
- Ardell J.L., Rajendran P.S., Nier H.A., KenKnight B.H., Armour J.A. Central-peripheral neural network interactions evoked by vagus nerve stimulation: functional consequences on control of cardiac function. Am. J. Physiol. Heart Circ. Physiol. 2015;309:H1740–H1752. doi: 10.1152/ajpheart.00557.2015. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

